Scaffoldless tissue-engineered nerve conduit promotes peripheral nerve regeneration and functional recovery after tibial nerve injury in rats
- PMID: 29090000
- PMCID: PMC5649475
- DOI: 10.4103/1673-5374.215265
Scaffoldless tissue-engineered nerve conduit promotes peripheral nerve regeneration and functional recovery after tibial nerve injury in rats
Abstract
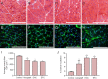
Damage to peripheral nerve tissue may cause loss of function in both the nerve and the targeted muscles it innervates. This study compared the repair capability of engineered nerve conduit (ENC), engineered fibroblast conduit (EFC), and autograft in a 10-mm tibial nerve gap. ENCs were fabricated utilizing primary fibroblasts and the nerve cells of rats on embryonic day 15 (E15). EFCs were fabricated utilizing primary fibroblasts only. Following a 12-week recovery, nerve repair was assessed by measuring contractile properties in the medial gastrocnemius muscle, distal motor nerve conduction velocity in the lateral gastrocnemius, and histology of muscle and nerve. The autografts, ENCs and EFCs reestablished 96%, 87% and 84% of native distal motor nerve conduction velocity in the lateral gastrocnemius, 100%, 44% and 44% of native specific force of medical gastrocnemius, and 63%, 61% and 67% of native medial gastrocnemius mass, respectively. Histology of the repaired nerve revealed large axons in the autograft, larger but fewer axons in the ENC repair, and many smaller axons in the EFC repair. Muscle histology revealed similar muscle fiber cross-sectional areas among autograft, ENC and EFC repairs. In conclusion, both ENCs and EFCs promoted nerve regeneration in a 10-mm tibial nerve gap repair, suggesting that the E15 rat nerve cells may not be necessary for nerve regeneration, and EFC alone can suffice for peripheral nerve injury repair.
Keywords: fibroblasts; nerve regeneration; neural cells; neural conduit; peripheral nerve repair; tissue engineering.
Conflict of interest statement
Conflicts of interest: None declared.
Figures


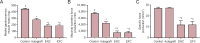

References
-
- Akassoglou K, Yu WM, Akpinar P, Strickland S. Fibrin inhibits peripheral nerve remyelination by regulating Schwann cell differentiation. Neuron. 2002;33:861–875. - PubMed
-
- Alluin O, Wittmann C, Marqueste T, Chabas JF, Garcia S, Lavaut MN, Guinard D, Feron F, Decherchi P. Functional recovery after peripheral nerve injury and implantation of a collagen guide. Biomaterials. 2009;30:363–373. - PubMed
-
- Archibald SJ, Krarup C, Shefner J, Li ST, Madison RD. A collagen-based nerve guide conduit for peripheral nerve repair: an electrophysiological study of nerve regeneration in rodents and nonhuman primates. J Comp Neurol. 1991;306:685–696. - PubMed
-
- Bini TB, Gao S, Xu X, Wang S, Ramakrishna S, Leong KW. Peripheral nerve regeneration by microbraided poly(L-lactide-co-glycolide) biodegradable polymer fibers. J Biomed Mater Res A. 2004;68:286–295. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

