Current understanding of the molecular mechanisms in Parkinson's disease: Targets for potential treatments
- PMID: 29090092
- PMCID: PMC5655877
- DOI: 10.1186/s40035-017-0099-z
Current understanding of the molecular mechanisms in Parkinson's disease: Targets for potential treatments
Abstract
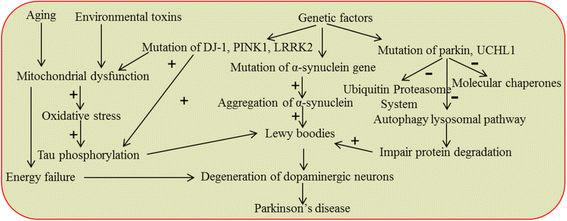
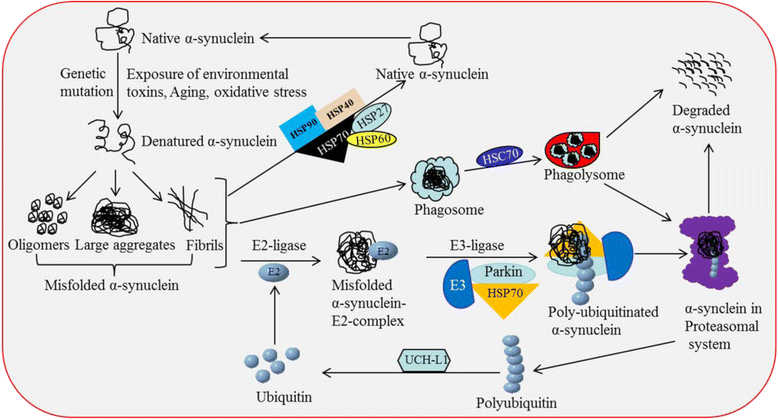
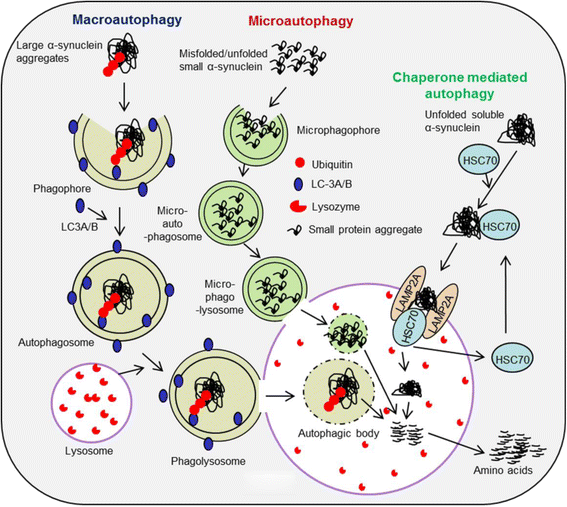
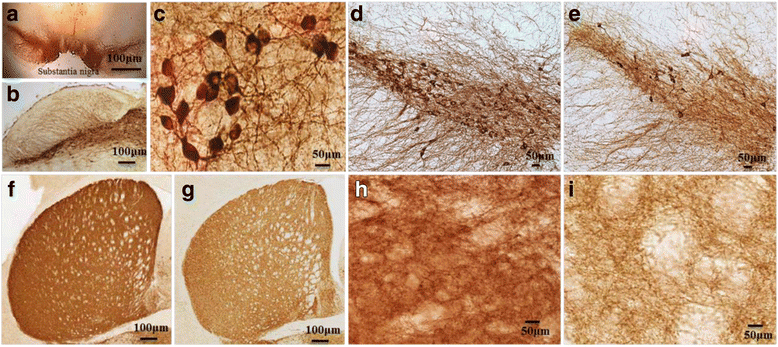
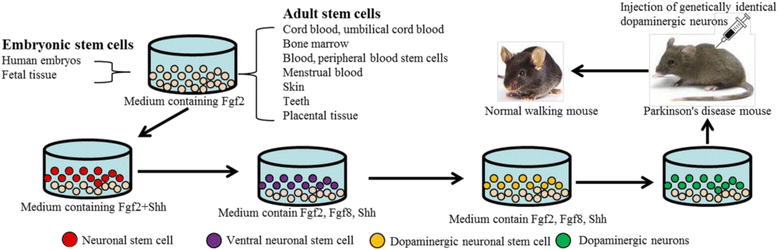
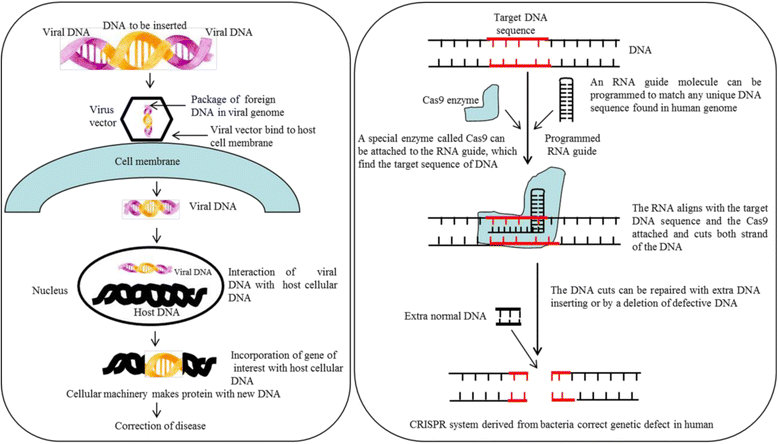
Gradual degeneration and loss of dopaminergic neurons in the substantia nigra, pars compacta and subsequent reduction of dopamine levels in striatum are associated with motor deficits that characterize Parkinson's disease (PD). In addition, half of the PD patients also exhibit frontostriatal-mediated executive dysfunction, including deficits in attention, short-term working memory, speed of mental processing, and impulsivity. The most commonly used treatments for PD are only partially or transiently effective and are available or applicable to a minority of patients. Because, these therapies neither restore the lost or degenerated dopaminergic neurons, nor prevent or delay the disease progression, the need for more effective therapeutics is critical. In this review, we provide a comprehensive overview of the current understanding of the molecular signaling pathways involved in PD, particularly within the context of how genetic and environmental factors contribute to the initiation and progression of this disease. The involvement of molecular chaperones, autophagy-lysosomal pathways, and proteasome systems in PD are also highlighted. In addition, emerging therapies, including pharmacological manipulations, surgical procedures, stem cell transplantation, gene therapy, as well as complementary, supportive and rehabilitation therapies to prevent or delay the progression of this complex disease are reviewed.
Keywords: Cell therapy; Molecular chaperones; Neurodegeneration; Parkinson’s disease; Protein misfolding.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors agreed to publish this article.
Competing interests
The authors declare that they have no competing interests.
Figures




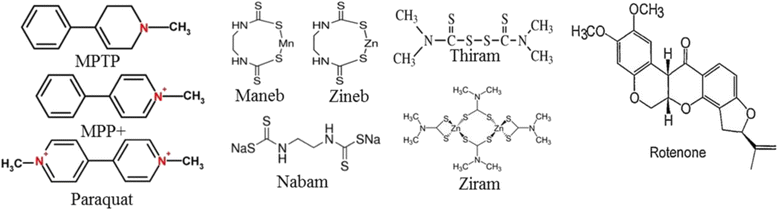
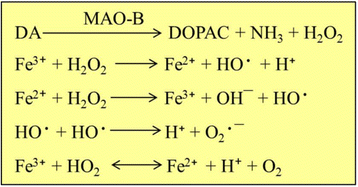



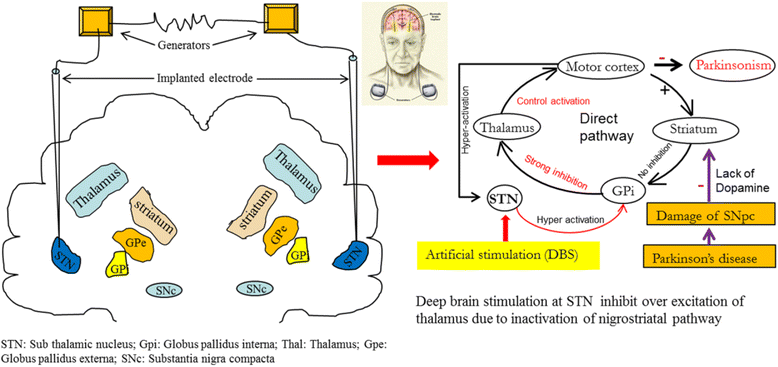
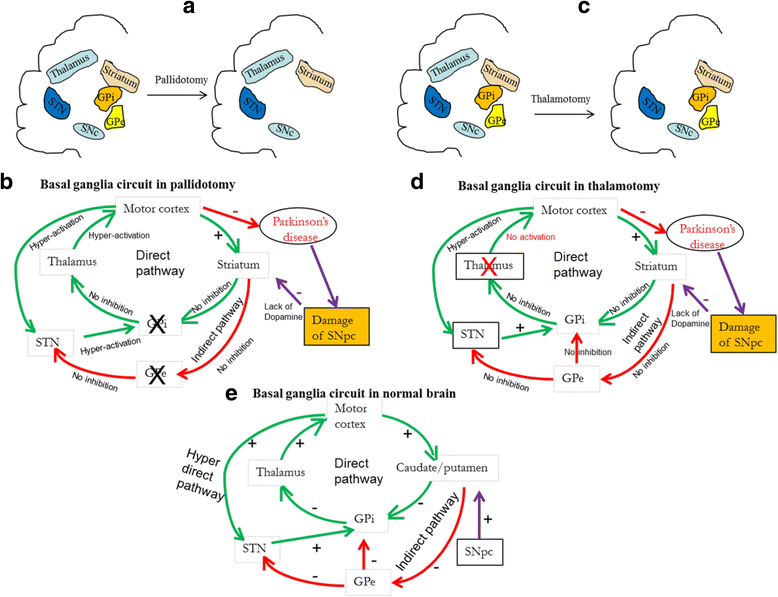
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

