Metabolism of Citrate and Other Carboxylic Acids in Erythrocytes As a Function of Oxygen Saturation and Refrigerated Storage
- PMID: 29090212
- PMCID: PMC5650965
- DOI: 10.3389/fmed.2017.00175
Metabolism of Citrate and Other Carboxylic Acids in Erythrocytes As a Function of Oxygen Saturation and Refrigerated Storage
Abstract
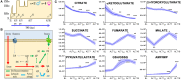
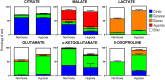
State-of-the-art proteomics technologies have recently helped to elucidate the unanticipated complexity of red blood cell metabolism. One recent example is citrate metabolism, which is catalyzed by cytosolic isoforms of Krebs cycle enzymes that are present and active in mature erythrocytes and was determined using quantitative metabolic flux analysis. In previous studies, we reported significant increases in glycolytic fluxes in red blood cells exposed to hypoxia in vitro or in vivo, an observation relevant to transfusion medicine owing to the potential benefits associated with hypoxic storage of packed red blood cells. Here, using a combination of steady state and quantitative tracing metabolomics experiments with 13C1,2,3-glucose, 13C6-citrate, 13C515N2-glutamine, and 13C1-aspartate via ultra-high performance liquid chromatography coupled on line with mass spectrometry, we observed that hypoxia in vivo and in vitro promotes consumption of citrate and other carboxylates. These metabolic reactions are theoretically explained by the activity of cytosolic malate dehydrogenase 1 and isocitrate dehydrogenase 1 (abundantly represented in the red blood cell proteome), though moonlighting functions of additional enzymes cannot be ruled out. These observations enhance understanding of red blood cell metabolic responses to hypoxia, which could be relevant to understand systemic physiological and pathological responses to high altitude, ischemia, hemorrhage, sepsis, pulmonary hypertension, or hemoglobinopathies. Results from this study will also inform the design and testing of novel additive solutions that optimize red blood cell storage under oxygen-controlled conditions.
Keywords: flux analysis; hypoxia; mass spectrometry; metabolomics; tracing experiments.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

