Utility of Different Adherence Measures for PrEP: Patterns and Incremental Value
- PMID: 29090394
- PMCID: PMC5878836
- DOI: 10.1007/s10461-017-1951-y
Utility of Different Adherence Measures for PrEP: Patterns and Incremental Value
Abstract
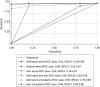
Measuring PrEP adherence remains challenging. In 2009-2010, the International AIDS Vaccine Initiative randomized phase II trial participants to daily tenofovir disoproxil fumarate/emtricitabine or placebo in Uganda and Kenya. Adherence was measured by electronic monitoring (EM), self-report (SR), and drug concentrations in plasma and hair. Each adherence measure was categorised as low, moderate, or high and also considered continuously; the incremental value of combining measures was determined. Forty-five participants were followed over 4 months. Discrimination for EM adherence by area under receiver operating curves (AROC) was poor for SR (0.53) and best for hair (AROC 0.85). When combining hair with plasma or hair with self-report, discrimination was improved (AROC > 0.9). Self-reported adherence was of low utility by itself. Hair level was the single best PK measure to predict EM-assessed adherence; the other measurements had lower discrimination values. Combining short-term (plasma) and long-term (hair) metrics could be useful to assess patterns of drug-taking in the context of PrEP.
Keywords: Hair; Plasma; PrEP drug-taking patterns of adherence electronic monitoring.
Conflict of interest statement
Conflict of interest
All authors declare that they have no conflict of interest.
Informed Consent
All trial participants provided written informed consent before enrolment. At each site, the trials were approved by the respective ethical committees including the Uganda Virus Research Institute Research and Ethics Committee, Uganda National Council for Science and Technology and the National Drug Authority, Kenyatta National Hospital Ethics Review Committee and the Kenya Medical Research Institute Ethics Review Committee.
Figures
References
-
- UNAIDS. Global AIDS update. 2016.
-
- Choopanya K, Martin M, Suntharasamai P, Sangkum U, Mock PA, Leethochawalit M, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2013;381(9883):2083–2090. doi: 10.1016/S0140-6736(13)61127-7. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous