A novel biosensor with high signal-to-noise ratio for real-time measurement of dopamine levels in vivo
- PMID: 29090830
- PMCID: PMC5873456
- DOI: 10.1002/jnr.24193
A novel biosensor with high signal-to-noise ratio for real-time measurement of dopamine levels in vivo
Abstract
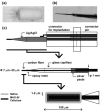
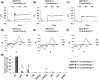
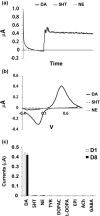
Fast-scan cyclic voltammetry (FSCV) is an established method for measuring dopamine (DA) levels in the brain in real time. However, it is difficult to discriminate DA from other monoamines such as serotonin (5-hydroxytryptamine, 5-HT) and norepinephrine (NE). We report a novel DA-specific biosensor consisting of a carbon-fiber electrode coated with an ion-exchange membrane, a layer containing monoamine oxidase B, and a cellulose membrane. We performed FSCV using the probe to monitor the amount of DA in vitro and in vivo. First, we measured currents in vitro in phosphate-buffered saline as we added one micromole each of DA, 5-HT, and NE. The results confirmed that the biosensor selectively detected DA. Next, we implanted the probe in the striatum of male rats to investigate whether it could selectively detect changes in the DA content in vivo. The probe detected both the tonic change induced by methamphetamine administration and the phasic change induced by electrical stimulation of the medial forebrain bundle. In contrast, the electrode in the 6-hydroxydopamine-lesioned striatum did not respond to systemic selective serotonin or serotonin/norepinephrine reuptake inhibitors, confirming its selectivity. Furthermore, the probe in the striatum could still detect changes in the DA level 1 week after electrode implantation. The results suggest that the novel biosensor can measure real-time changes in DA levels in vivo with a relatively high signal-to-noise ratio.
Keywords: electrode; fast-scan cyclic voltammetry; monoamine oxidase.
© 2017 The Authors Journal of Neuroscience Research Published by Wiley Periodicals, Inc.
Figures




References
-
- Alexander, G. E. , DeLong, M. R. , & Strick, P. L. (1986). Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual Review of Neuroscience, 9, 357–381. - PubMed
-
- Anastassiou, C. A. , Patel, B. A. , Arundell, M. , Yeoman, M. S. , Parker, K. H. , & O'Hare, D. (2006). Subsecond voltammetric separation between dopamine and serotonin in the presence of ascorbate. Analytical Chemistry, 78, 6990–6998. - PubMed
-
- Bourdy, R. , & Barrot, M. (2012). A new control center for dopaminergic systems: Pulling the VTA by the tail. Trends in Neurosciences, 35, 681–690. - PubMed
-
- Burmeister, J. J. , Pomerleau, F. , Huettl, P. , Gash, C. R. , Werner, C. E. , Bruno, J. P. , & Gerhardt, G. A. (2008). Ceramic‐based multisite microelectrode arrays for simultaneous measures of choline and acetylcholine in CNS. Biosensors & Bioelectronics, 23, 1382–1389. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

