Characterization of Solubilizing Nanoaggregates Present in Different Versions of Simulated Intestinal Fluid
- PMID: 29090933
- PMCID: PMC6209315
- DOI: 10.1021/acs.jpcb.7b08622
Characterization of Solubilizing Nanoaggregates Present in Different Versions of Simulated Intestinal Fluid
Abstract
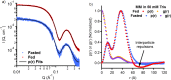
The absorption of hydrophobic drugs and nutrients from the intestine is principally determined by the amount that can be dissolved by the endogenous fluids present in the gut. Human intestinal fluids (HIFs) comprise a complex mixture of bile salts, phospholipids, steroids and glycerides that vary in composition in the fed and fasted state and between subjects. A number of simulated intestinal fluid (SIF) compositions have been developed to mimic fasted and fed state intestinal conditions and allow the in vitro determination of drug solubility as a proxy for the maximum dissolved concentration it is possible to reach. In particular these solvents are used during the development of lipophilic and poorly water-soluble drugs but questions remain around the differences that may arise from the source and methods of preparation of these fluids. In this work, a range of SIFs were studied using small-angle X-ray scattering (SAXS), cryogenic-transmission electron microscopy (cryo-TEM) and molecular dynamics (MD) simulations in order to analyze their structures. In-house prepared SIFs based on sodium taurodeoxycholate (NaTDC) and 1,2-dioleoyl-sn-glycero-3-phosphatidylcholine (DOPC) formed oblate ellipsoidal micelles irrespective of lipid concentration and preparation conditions. In contrast, commercially available SIFs based on sodium taurocholate and lecithin formed prolate ellipsoidal micelles in the fed state and vesicles in the fasted state. These structural variations are the likely reason for the dramatic differences sometimes observed in the solubility enhancements for hydrophobic drugs, nutrients and digestion products when using different SIFs. However, the structural homogeneity of the NaTDC/DOPC micelles makes them ideal candidates for standardizing SIF formulations as the structures of the solubilizing nanoaggregates therein are not sensitive to the preparation method.
Conflict of interest statement
The authors declare no competing financial interest.
Figures







References
-
- Madenci D.; Egelhaaf S. U. Self-Assembly in Aqueous Bile Salt Solutions. Curr. Opin. Colloid Interface Sci. 2010, 15 (1–2), 109–115. 10.1016/j.cocis.2009.11.010. - DOI
-
- Hogan A.; Ealick S. E.; Bugg C. E.; Barnes S. Aggregation Patterns of Bile Salts: Crystal Structure of Calcium Cholate Chloride Heptahydrate. J. Lipid Res. 1984, 25 (8), 791–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

