Fyn-dependent phosphorylation of PlexinA1 and PlexinA2 at conserved tyrosines is essential for zebrafish eye development
- PMID: 29091353
- PMCID: PMC5760361
- DOI: 10.1111/febs.14313
Fyn-dependent phosphorylation of PlexinA1 and PlexinA2 at conserved tyrosines is essential for zebrafish eye development
Abstract
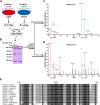
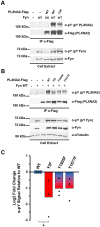
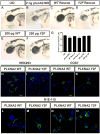
Plexins (Plxns) are semaphorin (Sema) receptors that play important signaling roles, particularly in the developing nervous system and vasculature. Sema-Plxn signaling regulates cellular processes such as cytoskeletal dynamics, proliferation, and differentiation. However, the receptor-proximal signaling mechanisms driving Sema-Plxn signal transduction are only partially understood. Plxn tyrosine phosphorylation is thought to play an important role in these signaling events as receptor and nonreceptor tyrosine kinases have been shown to interact with Plxn receptors. The Src family kinase Fyn can induce the tyrosine phosphorylation of PlxnA1 and PlxnA2. However, the Fyn-dependent phosphorylation sites on these receptors have not been identified. Here, using mass spectrometry-based approaches, we have identified highly conserved, Fyn-induced PlexinA (PlxnA) tyrosine phosphorylation sites. Mutation of these sites to phenylalanine results in significantly decreased Fyn-dependent PlxnA tyrosine phosphorylation. Furthermore, in contrast to wild-type human PLXNA2 mRNA, mRNA harboring these point mutations cannot rescue eye developmental defects when coinjected with a plxnA2 morpholino in zebrafish embryos. Together these data suggest that Fyn-dependent phosphorylation at two critical tyrosines is a key feature of vertebrate PlxnA1 and PlxnA2 signal transduction.
Keywords: Fyn; mass spectrometry; phosphorylation; plexin; semaphorin.
© 2017 Federation of European Biochemical Societies.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

