Biochemometrics to Identify Synergists and Additives from Botanical Medicines: A Case Study with Hydrastis canadensis (Goldenseal)
- PMID: 29091439
- PMCID: PMC6251718
- DOI: 10.1021/acs.jnatprod.7b00654
Biochemometrics to Identify Synergists and Additives from Botanical Medicines: A Case Study with Hydrastis canadensis (Goldenseal)
Abstract
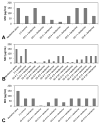
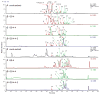
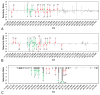
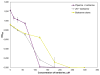
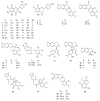
A critical challenge in the study of botanical natural products is the difficulty of identifying multiple compounds that may contribute additively, synergistically, or antagonistically to biological activity. Herein, it is demonstrated how combining untargeted metabolomics with synergy-directed fractionation can be effective toward accomplishing this goal. To demonstrate this approach, an extract of the botanical goldenseal ( Hydrastis canadensis) was fractionated and tested for its ability to enhance the antimicrobial activity of the alkaloid berberine (4) against the pathogenic bacterium Staphylococcus aureus. Bioassay data were combined with untargeted mass spectrometry-based metabolomics data sets (biochemometrics) to produce selectivity ratio (SR) plots, which visually show which extract components are most strongly associated with the biological effect. Using this approach, the new flavonoid 3,3'-dihydroxy-5,7,4'-trimethoxy-6,8- C-dimethylflavone (29) was identified, as were several flavonoids known to be active. When tested in combination with 4, 29 lowered the IC50 of 4 from 132.2 ± 1.1 μM to 91.5 ± 1.1 μM. In isolation, 29 did not demonstrate antimicrobial activity. The current study highlights the importance of fractionation when utilizing metabolomics for identifying bioactive components from botanical extracts and demonstrates the power of SR plots to help merge and interpret complex biological and chemical data sets.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

