Bosutinib Versus Imatinib for Newly Diagnosed Chronic Myeloid Leukemia: Results From the Randomized BFORE Trial
- PMID: 29091516
- PMCID: PMC5966023
- DOI: 10.1200/JCO.2017.74.7162
Bosutinib Versus Imatinib for Newly Diagnosed Chronic Myeloid Leukemia: Results From the Randomized BFORE Trial
Abstract
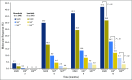
Purpose Bosutinib is a potent dual SRC/ABL kinase inhibitor approved for adults with Philadelphia chromosome-positive chronic myeloid leukemia (CML) resistant and /or intolerant to prior therapy. We assessed the efficacy and safety of bosutinib versus imatinib for first-line treatment of chronic-phase CML. Methods In this ongoing, multinational, phase III study, 536 patients with newly diagnosed chronic-phase CML were randomly assigned 1:1 to receive 400 mg of bosutinib once daily (n = 268) or imatinib (n = 268). Per protocol, efficacy was assessed in patients who were Philadelphia chromosome-positive with typical (e13a2/e14a2) transcripts (bosutinib, n = 246; imatinib, n = 241). Patients with Philadelphia chromosome-negative-/ BCR-ABL1-positive status and those with unknown Philadelphia chromosome status and/or atypical BCR-ABL1 transcript type were excluded from this population. Results The major molecular response (MMR) rate at 12 months (primary end point) was significantly higher with bosutinib versus imatinib (47.2% v 36.9%, respectively; P = .02), as was complete cytogenetic response (CCyR) rate by 12 months (77.2% v 66.4%, respectively; P = .0075). Cumulative incidence was favorable with bosutinib (MMR: hazard ratio, 1.34; P = .0173; CCyR: hazard ratio, 1.38; P < .001), with earlier response times. Four patients (1.6%) receiving bosutinib and six patients (2.5%) receiving imatinib experienced disease progression to accelerated/blast phase. Among treated patients, 22.0% of patients receiving bosutinib and 26.8% of patients receiving imatinib discontinued treatment, most commonly for drug-related toxicity (12.7% and 8.7%, respectively). Grade ≥ 3 diarrhea (7.8% v 0.8%) and increased ALT (19.0% v 1.5%) and AST (9.7% v 1.9%) levels were more common with bosutinib. Cardiac and vascular toxicities were uncommon. Conclusion Patients who received bosutinib had significantly higher rates of MMR and CCyR and achieved responses faster than those who received imatinib. Consistent with the known safety profile, GI events and transaminase elevations were more common with bosutinib. Results indicate bosutinib may be an effective first-line treatment for chronic-phase CML.
Trial registration: ClinicalTrials.gov NCT02130557.
Figures
Comment in
-
Front-Line Treatment Options for Chronic-Phase Chronic Myeloid Leukemia.J Clin Oncol. 2018 Jan 20;36(3):220-224. doi: 10.1200/JCO.2017.75.4663. Epub 2017 Dec 5. J Clin Oncol. 2018. PMID: 29206554
References
-
- Gambacorti-Passerini C, Antolini L, Mahon FX, et al. : Multicenter independent assessment of outcomes in chronic myeloid leukemia patients treated with imatinib. J Natl Cancer Inst 103:553-561, 2011 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

