The cathelicidin protein CRAMP is a potential atherosclerosis self-antigen in ApoE(-/-) mice
- PMID: 29091929
- PMCID: PMC5665601
- DOI: 10.1371/journal.pone.0187432
The cathelicidin protein CRAMP is a potential atherosclerosis self-antigen in ApoE(-/-) mice
Abstract
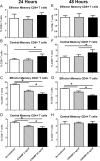
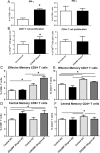
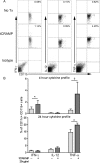
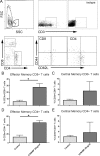
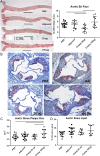
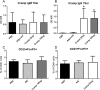
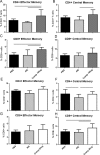
Auto-immunity is believed to contribute to inflammation in atherosclerosis. The antimicrobial peptide LL-37, a fragment of the cathelicidin protein precursor hCAP18, was previously identified as an autoantigen in psoriasis. Given the reported link between psoriasis and coronary artery disease, the biological relevance of the autoantigen to atherosclerosis was tested in vitro using a truncated (t) form of the mouse homolog of hCAP18, CRAMP, on splenocytes from athero-prone ApoE(-/-) mice. Stimulation with tCRAMP resulted in increased CD8+ T cells with Central Memory and Effector Memory phenotypes in ApoE(-/-) mice, differentially activated by feeding with normal chow or high fat diet. Immunization of ApoE(-/-) with different doses of the shortened peptide (Cramp) resulted in differential outcomes with a lower dose reducing atherosclerosis whereas a higher dose exacerbating the disease with increased neutrophil infiltration of the atherosclerotic plaques. Low dose Cramp immunization also resulted in increased splenic CD8+ T cell degranulation and reduced CD11b+CD11c+ conventional dendritic cells (cDCs), whereas high dose increased CD11b+CD11c+ cDCs. Our results identified CRAMP, the mouse homolog of hCAP-18, as a potential self-antigen involved in the immune response to atherosclerosis in the ApoE(-/-) mouse model.
Conflict of interest statement
Figures


References
-
- Nilsson J, Hansson GK. Autoimmunity in atherosclerosis: a protective response losing control? J Intern Med. 2008;263(5): 464–478. doi: 10.1111/j.1365-2796.2008.01945.x - DOI - PubMed
-
- Zhou X, Nicoletti A, Elhage R, Hansson GK. Transfer of CD4(+) T cells aggravates atherosclerosis in immunodeficient apolipoprotein E knockout mice. Circulation. 2000;102(24): 2919–2922. - PubMed
-
- Almanzar G, Ollinger R, Leuenberger J, Onestingel E, Rantner B, Zehm S, et al. Autoreactive HSP60 epitope-specific T-cells in early human atherosclerotic lesions. J Autoimmun. 2012;39: 441–450. doi: 10.1016/j.jaut.2012.07.006 - DOI - PMC - PubMed
-
- Nilsson J, Bjorkbacka H, Fredrikson GN. Apolipoprotein B100 autoimmunity and atherosclerosis—disease mechanisms and therapeutic potential. Curr Opin Lipidol. 2012;23(5): 422–428. doi: 10.1097/MOL.0b013e328356ec7c - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

