Characterization of male breast cancer: results of the EORTC 10085/TBCRC/BIG/NABCG International Male Breast Cancer Program
- PMID: 29092024
- PMCID: PMC5834077
- DOI: 10.1093/annonc/mdx651
Characterization of male breast cancer: results of the EORTC 10085/TBCRC/BIG/NABCG International Male Breast Cancer Program
Abstract
Background: Male breast cancer (BC) is rare, managed by extrapolation from female BC. The International Male BC Program aims to better characterize and manage this disease. We report the results of part I, a retrospective joint analysis of cases diagnosed during a 20-year period.
Methods: Patients with follow-up and tumor samples, treated between 1990 and 2010, in 93 centers/9 countries. Samples were centrally analyzed in three laboratories (the United Kingdom, the Netherlands and the United States).
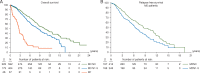
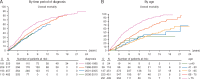
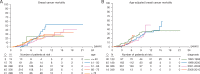
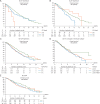
Results: Of 1822 patients enrolled, 1483 were analyzed; 63.5% were diagnosed between 2001 and 2010, 57 (5.1%) had metastatic disease (M1). Median age at diagnosis: 68.4 years. Of 1054 M0 cases, 56.2% were node-negative (N0) and 48.5% had T1 tumors; 4% had breast conserving surgery (BCS), 18% sentinel lymph-node biopsy; half received adjuvant radiotherapy; 29.8% (neo)adjuvant chemotherapy and 76.8% adjuvant endocrine therapy (ET), mostly tamoxifen (88.4%). Per central pathology, for M0 tumors: 84.8% ductal invasive carcinomas, 51.5% grade 2; 99.3% estrogen receptor (ER)-positive; 81.9% progesterone receptor (PR)-positive; 96.9% androgen receptor (AR)-positive [ER, PR or AR Allred score ≥3]; 61.1% Ki67 expression low (<14% positive cells); using immunohistochemistry (IHC) surrogates, 41.9% were Luminal-A-like, 48.6% Luminal-B-like/HER-2-negative, 8.7% HER-2-positive, 0.3% triple negative. Median follow-up: 8.2 years (0.0-23.8) for all, 7.2 years (0.0-23.2), for M0, 2.6 years (0.0-12.7) for M1 patients. A significant improvement over time was observed in age-corrected BC mortality. BC-specific-mortality was higher for men younger than 50 years. Better overall (OS) and recurrence-free survival (RFS) were observed for highly ER+ (P = 0.001), highly PR+ (P = 0.002), highly AR+ disease (P = 0.019). There was no association between OS/RFS and HER-2 status, Ki67, IHC subtypes nor grade.
Conclusions: Male BC is usually ER, PR and AR-positive, Luminal B-like/HER2-negative. Of note, 56% patients had T1 tumors but only 4% had BCS. ER was highly positive in >90% of cases but only 77% received adjuvant ET. ER, PR and AR were associated with OS and RFS, whereas grade, Ki67 and IHC surrogates were not. Significant improvement in survival over time was observed.
© The Author 2017. Published by Oxford University Press on behalf of the European Society for Medical Oncology. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures
Comment in
-
Male breast cancer: pink ribbon blues.Ann Oncol. 2018 Feb 1;29(2):292-293. doi: 10.1093/annonc/mdx752. Ann Oncol. 2018. PMID: 29165550 No abstract available.
References
-
- Giordano SH, Cohen DS, Buzdar AU. et al. Breast carcinoma in men: a population-based study. Cancer 2004; 101(1): 51–57. - PubMed
-
- Speirs V, Shaaban AM.. The rising incidence of male breast cancer. Breast Cancer Res Treat 2009; 115(2): 429–430. - PubMed
-
- Evans DB, Crichlow RW.. Carcinoma of the male breast and Klinefelter's syndrome: is there an association? CA Cancer J Clin 1987; 37(4): 246–251. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

