Uncoupling Exercise Bioenergetics From Systemic Metabolic Homeostasis by Conditional Inactivation of Baf60 in Skeletal Muscle
- PMID: 29092888
- PMCID: PMC5741141
- DOI: 10.2337/db17-0367
Uncoupling Exercise Bioenergetics From Systemic Metabolic Homeostasis by Conditional Inactivation of Baf60 in Skeletal Muscle
Abstract
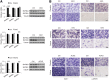
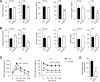
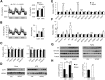
Impaired skeletal muscle energy metabolism is linked to the pathogenesis of insulin resistance and glucose intolerance in type 2 diabetes. The contractile and metabolic properties of myofibers exhibit a high degree of heterogeneity and plasticity. The regulatory circuitry underpinning skeletal muscle energy metabolism is critically linked to exercise endurance and systemic homeostasis. Recent work has identified the Baf60 subunits of the SWI/SNF chromatin-remodeling complex as powerful regulators of the metabolic gene programs. However, their role in integrating myofiber energy metabolism with exercise endurance and metabolic physiology remains largely unknown. In this study, we conditionally inactivated Baf60a, Baf60c, or both in mature skeletal myocytes to delineate their contribution to muscle bioenergetics and metabolic physiology. Our work revealed functional redundancy between Baf60a and Baf60c in maintaining oxidative and glycolytic metabolism in skeletal myofibers and exercise endurance. Unexpectedly, mice lacking these two factors in skeletal muscle were protected from diet-induced and age-associated metabolic disorders. Transcriptional profiling analysis identified the muscle thermogenic gene program and myokine secretion as key pathways that integrate myofiber metabolism with systemic energy balance. As such, Baf60 deficiency in skeletal muscle illustrates a surprising disconnect between exercise endurance and systemic metabolic homeostasis.
© 2017 by the American Diabetes Association.
Figures




References
-
- Schiaffino S. Fibre types in skeletal muscle: a personal account. Acta Physiol (Oxf) 2010;199:451–463 - PubMed
-
- Bassel-Duby R, Olson EN. Signaling pathways in skeletal muscle remodeling. Annu Rev Biochem 2006;75:19–37 - PubMed
-
- Berchtold MW, Brinkmeier H, Müntener M. Calcium ion in skeletal muscle: its crucial role for muscle function, plasticity, and disease. Physiol Rev 2000;80:1215–1265 - PubMed
-
- Egan B, Zierath JR. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab 2013;17:162–184 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

