Coordination and redox state-dependent structural changes of the heme-based oxygen sensor Af GcHK associated with intraprotein signal transduction
- PMID: 29092908
- PMCID: PMC5743068
- DOI: 10.1074/jbc.M117.817023
Coordination and redox state-dependent structural changes of the heme-based oxygen sensor Af GcHK associated with intraprotein signal transduction
Abstract
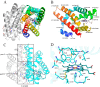
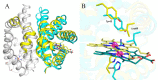
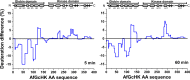
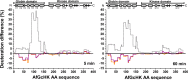
The heme-based oxygen sensor histidine kinase AfGcHK is part of a two-component signal transduction system in bacteria. O2 binding to the Fe(II) heme complex of its N-terminal globin domain strongly stimulates autophosphorylation at His183 in its C-terminal kinase domain. The 6-coordinate heme Fe(III)-OH- and -CN- complexes of AfGcHK are also active, but the 5-coordinate heme Fe(II) complex and the heme-free apo-form are inactive. Here, we determined the crystal structures of the isolated dimeric globin domains of the active Fe(III)-CN- and inactive 5-coordinate Fe(II) forms, revealing striking structural differences on the heme-proximal side of the globin domain. Using hydrogen/deuterium exchange coupled with mass spectrometry to characterize the conformations of the active and inactive forms of full-length AfGcHK in solution, we investigated the intramolecular signal transduction mechanisms. Major differences between the active and inactive forms were observed on the heme-proximal side (helix H5), at the dimerization interface (helices H6 and H7 and loop L7) of the globin domain and in the ATP-binding site (helices H9 and H11) of the kinase domain. Moreover, separation of the sensor and kinase domains, which deactivates catalysis, increased the solvent exposure of the globin domain-dimerization interface (helix H6) as well as the flexibility and solvent exposure of helix H11. Together, these results suggest that structural changes at the heme-proximal side, the globin domain-dimerization interface, and the ATP-binding site are important in the signal transduction mechanism of AfGcHK. We conclude that AfGcHK functions as an ensemble of molecules sampling at least two conformational states.
Keywords: bacterial protein kinase; crystal structure; globin; heme-containing oxygen sensor; histidine kinase; hydrogen-deuterium exchange; signal transduction; two component signal transduction system.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures





References
-
- Gushchin I., Melnikov I., Polovinkin V., Ishchenko A., Yuzhakova A., Buslaev P., Bourenkov G., Grudinin S., Round E., Balandin T., Borshchevskiy V., Willbold D., Leonard G., Büldt G., Popov A., and Gordeliy V. (2017) Mechanism of transmembrane signaling by sensor histidine kinases. Science 10.1126/science.aah6345 - DOI - PubMed
-
- Abriata L. A., Albanesi D., Dal Peraro M., and de Mendoza D. (2017) Signal sensing and transduction by histidine kinases as unveiled through studies on a temperature sensor. Acc. Chem. Res. 50, 1359–1366 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

