Genome-Wide Meta-Analysis Unravels Interactions between Magnesium Homeostasis and Metabolic Phenotypes
- PMID: 29093028
- PMCID: PMC5748908
- DOI: 10.1681/ASN.2017030267
Genome-Wide Meta-Analysis Unravels Interactions between Magnesium Homeostasis and Metabolic Phenotypes
Abstract
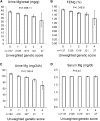
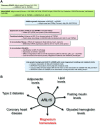
Magnesium (Mg2+) homeostasis is critical for metabolism. However, the genetic determinants of the renal handling of Mg2+, which is crucial for Mg2+ homeostasis, and the potential influence on metabolic traits in the general population are unknown. We obtained plasma and urine parameters from 9099 individuals from seven cohorts, and conducted a genome-wide meta-analysis of Mg2+ homeostasis. We identified two loci associated with urinary magnesium (uMg), rs3824347 (P=4.4×10-13) near TRPM6, which encodes an epithelial Mg2+ channel, and rs35929 (P=2.1×10-11), a variant of ARL15, which encodes a GTP-binding protein. Together, these loci account for 2.3% of the variation in 24-hour uMg excretion. In human kidney cells, ARL15 regulated TRPM6-mediated currents. In zebrafish, dietary Mg2+ regulated the expression of the highly conserved ARL15 ortholog arl15b, and arl15b knockdown resulted in renal Mg2+ wasting and metabolic disturbances. Finally, ARL15 rs35929 modified the association of uMg with fasting insulin and fat mass in a general population. In conclusion, this combined observational and experimental approach uncovered a gene-environment interaction linking Mg2+ deficiency to insulin resistance and obesity.
Keywords: Gene-environment interaction; Genetic determinants; Magnesium homeostasis; Metabolic syndrome; Tubular transport; zebrafish.
Copyright © 2018 by the American Society of Nephrology.
Figures






References
-
- de Baaij JHF, Hoenderop JGJ, Bindels RJM: Magnesium in man: Implications for health and disease. Physiol Rev 95: 1–46, 2015 - PubMed
-
- Lopez-Ridaura R, Willett WC, Rimm EB, Liu S, Stampfer MJ, Manson JE, Hu FB: Magnesium intake and risk of type 2 diabetes in men and women. Diabetes Care 27: 134–140, 2004 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

