Glycan Shield and Fusion Activation of a Deltacoronavirus Spike Glycoprotein Fine-Tuned for Enteric Infections
- PMID: 29093093
- PMCID: PMC5790929
- DOI: 10.1128/JVI.01628-17
Glycan Shield and Fusion Activation of a Deltacoronavirus Spike Glycoprotein Fine-Tuned for Enteric Infections
Abstract
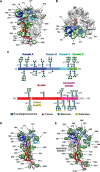
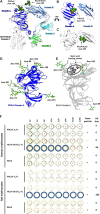
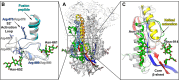
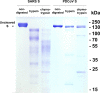
Coronaviruses recently emerged as major human pathogens causing outbreaks of severe acute respiratory syndrome and Middle East respiratory syndrome. They utilize the spike (S) glycoprotein anchored in the viral envelope to mediate host attachment and fusion of the viral and cellular membranes to initiate infection. The S protein is a major determinant of the zoonotic potential of coronaviruses and is also the main target of the host humoral immune response. We report here the 3.5-Å-resolution cryo-electron microscopy structure of the S glycoprotein trimer from the pathogenic porcine deltacoronavirus (PDCoV), which belongs to the recently identified Deltacoronavirus genus. Structural and glycoproteomics data indicate that the glycans of PDCoV S are topologically conserved compared with the human respiratory coronavirus NL63 S, resulting in similar surface areas being shielded from neutralizing antibodies and implying that both viruses are under comparable immune pressure in their respective hosts. The structure further reveals a shortened S2' activation loop, containing a reduced number of basic amino acids, which participates in rendering the spike largely protease resistant. This property distinguishes PDCoV S from recently characterized betacoronavirus S proteins and suggests that the S protein of enterotropic PDCoV has evolved to tolerate the protease-rich environment of the small intestine and to fine-tune its fusion activation to avoid premature triggering and reduction of infectivity.IMPORTANCE Coronaviruses use transmembrane S glycoprotein trimers to promote host attachment and fusion of the viral and cellular membranes. We determined a near-atomic-resolution cryo-electron microscopy structure of the S ectodomain trimer from the pathogenic PDCoV, which is responsible for diarrhea in piglets and has had devastating consequences for the swine industry worldwide. Structural and glycoproteomics data reveal that PDCoV S is decorated with 78 N-linked glycans obstructing the protein surface to limit accessibility to neutralizing antibodies in a way reminiscent of what has recently been described for a human respiratory coronavirus. PDCoV S is largely protease resistant, which distinguishes it from most other characterized coronavirus S glycoproteins and suggests that enteric coronaviruses have evolved to fine-tune fusion activation in the protease-rich environment of the small intestine of infected hosts.
Keywords: coronaviruses; cryo-EM; fusion proteins.
Copyright © 2018 American Society for Microbiology.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

