The immunopeptidomic landscape of ovarian carcinomas
- PMID: 29093164
- PMCID: PMC5699044
- DOI: 10.1073/pnas.1707658114
The immunopeptidomic landscape of ovarian carcinomas
Abstract
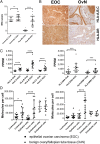
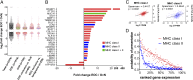
Immunotherapies, particularly checkpoint inhibitors, have set off a revolution in cancer therapy by releasing the power of the immune system. However, only little is known about the antigens that are essentially presented on cancer cells, capable of exposing them to immune cells. Large-scale HLA ligandome analysis has enabled us to exhaustively characterize the immunopeptidomic landscape of epithelial ovarian cancers (EOCs). Additional comparative profiling with the immunopeptidome of a variety of benign sources has unveiled a multitude of ovarian cancer antigens (MUC16, MSLN, LGALS1, IDO1, KLK10) to be presented by HLA class I and class II molecules exclusively on ovarian cancer cells. Most strikingly, ligands derived from mucin 16 and mesothelin, a molecular axis of prognostic importance in EOC, are prominent in a majority of patients. Differential gene-expression analysis has allowed us to confirm the relevance of these targets for EOC and further provided important insights into the relationship between gene transcript levels and HLA ligand presentation.
Keywords: immunopeptidome; immunotherapy; mesothelin; mucin 16; ovarian cancer.
Copyright © 2017 the Author(s). Published by PNAS.
Conflict of interest statement
Conflict of interest statement: H.-G.R. is a shareholder of Immatics Biotechnologies, Tübingen, and CureVac GmbH, Tübingen. H.S. and D.J.K. are employees of Immatics Biotechnologies GmbH. The authors declare that Immatics did not provide neither financial nor scientific support in any direct relation to this manuscript or the underlying studies, and was not involved in data collection, analysis, or decision to publish.
Figures


References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. - PubMed
-
- Herzog TJ, Pothuri B. Ovarian cancer: A focus on management of recurrent disease. Nat Clin Pract Oncol. 2006;3:604–611. - PubMed
-
- Mirza MR, et al. ENGOT-OV16/NOVA Investigators Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375:2154–2164. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

