Human vaccination against RH5 induces neutralizing antimalarial antibodies that inhibit RH5 invasion complex interactions
- PMID: 29093263
- PMCID: PMC5752323
- DOI: 10.1172/jci.insight.96381
Human vaccination against RH5 induces neutralizing antimalarial antibodies that inhibit RH5 invasion complex interactions
Abstract
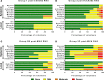
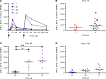
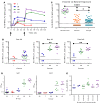
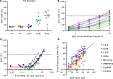
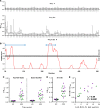
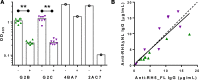
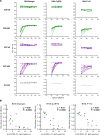
The development of a highly effective vaccine remains a key strategic goal to aid the control and eventual eradication of Plasmodium falciparum malaria. In recent years, the reticulocyte-binding protein homolog 5 (RH5) has emerged as the most promising blood-stage P. falciparum candidate antigen to date, capable of conferring protection against stringent challenge in Aotus monkeys. We report on the first clinical trial to our knowledge to assess the RH5 antigen - a dose-escalation phase Ia study in 24 healthy, malaria-naive adult volunteers. We utilized established viral vectors, the replication-deficient chimpanzee adenovirus serotype 63 (ChAd63), and the attenuated orthopoxvirus modified vaccinia virus Ankara (MVA), encoding RH5 from the 3D7 clone of P. falciparum. Vaccines were administered i.m. in a heterologous prime-boost regimen using an 8-week interval and were well tolerated. Vaccine-induced anti-RH5 serum antibodies exhibited cross-strain functional growth inhibition activity (GIA) in vitro, targeted linear and conformational epitopes within RH5, and inhibited key interactions within the RH5 invasion complex. This is the first time to our knowledge that substantial RH5-specific responses have been induced by immunization in humans, with levels greatly exceeding the serum antibody responses observed in African adults following years of natural malaria exposure. These data support the progression of RH5-based vaccines to human efficacy testing.
Conflict of interest statement
Figures

References
-
- WHO. World Malaria Report 2015. Geneva, Switzerland: World Health Organization; 2015. http://www.who.int/malaria/publications/world-malaria-report-2015/report... Accessed October 10, 2017.
-
- Moorthy VS, Newman RD, Okwo-Bele JM. Malaria vaccine technology roadmap. Lancet. 2013;382(9906):1700–1701. - PubMed
-
- Halbroth BR, Draper SJ. Recent developments in malaria vaccinology. Adv Parasitol. 2015;88:1–49. - PubMed
-
- Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet. 2015;386(9988):31–45. doi: 10.1016/S0140-6736(15)60721-8. RTS,S Clinical Trials Partnership. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

