T cells presenting viral antigens or autoantigens induce cytotoxic T cell anergy
- PMID: 29093272
- PMCID: PMC5752293
- DOI: 10.1172/jci.insight.96173
T cells presenting viral antigens or autoantigens induce cytotoxic T cell anergy
Abstract
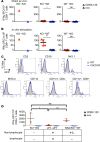
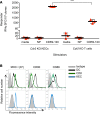
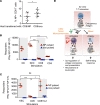
In the course of modeling the naturally occurring tumor immunity seen in patients with paraneoplastic cerebellar degeneration (PCD), we discovered an unexpectedly high threshold for breaking CD8+ cytotoxic T cell (CTL) tolerance to the PCD autoantigen, CDR2. While CDR2 expression was previously found to be strictly restricted to immune-privileged cells (cerebellum, testes, and tumors), unexpectedly we have found that T cells also express CDR2. This expression underlies inhibition of CTL activation; CTLs that respond to epithelial cells expressing CDR2 fail to respond to T cells expressing CDR2. This was a general phenomenon, as T cells presenting influenza (flu) antigen also fail to activate otherwise potent flu-specific CTLs either in vitro or in vivo. Moreover, transfer of flu peptide-pulsed T cells into flu-infected mice inhibits endogenous flu-specific CTLs. Our finding that T cells serve as a site of immune privilege, inhibiting effector CTL function, uncovers an autorepressive loop with general biologic and clinical relevance.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

