Blood meal acquisition enhances arbovirus replication in mosquitoes through activation of the GABAergic system
- PMID: 29093445
- PMCID: PMC5665997
- DOI: 10.1038/s41467-017-01244-6
Blood meal acquisition enhances arbovirus replication in mosquitoes through activation of the GABAergic system
Abstract
Mosquitoes are hematophagous insects that carry-on and transmit many human viruses. However, little information is available regarding the common mechanisms underlying the infection of mosquitoes by these viruses. In this study, we reveal that the hematophagous nature of mosquitoes contributes to arboviral infection after a blood meal, which suppresses antiviral innate immunity by activating the GABAergic pathway. dsRNA-mediated interruption of the GABA signaling and blockage of the GABAA receptor by the specific inhibitors both significantly impaired arbovirus replication. Consistently, inoculation of GABA enhanced arboviral infection, indicating that GABA signaling facilitates the arboviral infection of mosquitoes. The ingestion of blood by mosquitoes resulted in robust GABA production from glutamic acid derived from blood protein digestion. The oral introduction of glutamic acid increased virus acquisition by mosquitoes via activation of the GABAergic system. Our study reveals that blood meals enhance arbovirus replication in mosquitoes through activation of the GABAergic system.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

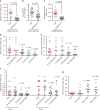
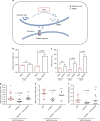
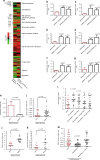
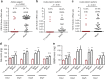
References
-
- Caraballo H, King K. Emergency department management of mosquito-borne illness: malaria, dengue, and West Nile virus. Emerg. Med. Practice. 2014;16:1–23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

