Retinoic acid signaling maintains epithelial and mesenchymal progenitors in the developing mouse ureter
- PMID: 29093497
- PMCID: PMC5665985
- DOI: 10.1038/s41598-017-14790-2
Retinoic acid signaling maintains epithelial and mesenchymal progenitors in the developing mouse ureter
Abstract
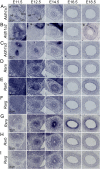
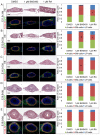
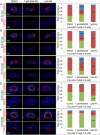
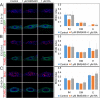
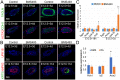
The differentiated cell types of the mature ureter arise from the distal ureteric bud epithelium and its surrounding mesenchyme. Uncommitted epithelial cells first become intermediate cells from which both basal and superficial cells develop. Mesenchymal progenitors give rise to separated layers of adventitial fibrocytes, smooth muscle cells and lamina propria fibrocytes. How progenitor expansion and differentiation are balanced is poorly understood. Here, we addressed the role of retinoic acid (RA) signaling in these programs. Using expression analysis of components and target genes, we show that pathway activity is restricted to the mesenchymal and epithelial progenitor pools. Inhibition of RA signaling in ureter explant cultures resulted in tissue hypoplasia with a relative expansion of smooth muscle cells at the expense of lamina propria fibroblasts in the mesenchyme, and of superficial cells at the expense of intermediate cells in the ureteric epithelium. Administration of RA led to a slight reduction of smooth muscle cells, and almost completely prevented differentiation of intermediate cells into basal and superficial cells. We identified cellular programs and transcriptional targets of RA signaling that may account for this activity. We conclude that RA signaling is required and sufficient to maintain mesenchymal and epithelial progenitors in early ureter development.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Velardo, J. T. In The ureter (ed. H. Bergman. (Springer-Verlag, 1981).
-
- Yu J, Carroll TJ, McMahon AP. Sonic hedgehog regulates proliferation and differentiation of mesenchymal cells in the mouse metanephric kidney. Development. 2002;129:5301–5312. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

