A phase I vaccination study with dendritic cells loaded with NY-ESO-1 and α-galactosylceramide: induction of polyfunctional T cells in high-risk melanoma patients
- PMID: 29094183
- PMCID: PMC11028320
- DOI: 10.1007/s00262-017-2085-9
A phase I vaccination study with dendritic cells loaded with NY-ESO-1 and α-galactosylceramide: induction of polyfunctional T cells in high-risk melanoma patients
Abstract
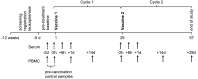
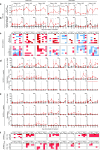
Vaccines that elicit targeted tumor antigen-specific T-cell responses have the potential to be used as adjuvant therapy in patients with high risk of relapse. However, the responses induced by vaccines in cancer patients have generally been disappointing. To improve vaccine function, we investigated the possibility of exploiting the immunostimulatory capacity of type 1 Natural killer T (NKT) cells, a cell type enriched in lymphoid tissues that can trigger improved antigen-presenting function in dendritic cells (DCs). In this phase I dose escalation study, we treated eight patients with high-risk surgically resected stage II-IV melanoma with intravenous autologous monocyte-derived DCs loaded with the NKT cell agonist α-GalCer and peptides derived from the cancer testis antigen NY-ESO-1. Two synthetic long peptides spanning defined immunogenic regions of the NY-ESO-1 sequence were used. This therapy proved to be safe and immunologically effective, inducing increases in circulating NY-ESO-1-specific T cells that could be detected directly ex vivo in seven out of eight patients. These responses were achieved using as few as 5 × 105 peptide-loaded cells per dose. Analysis after in vitro restimulation showed increases in polyfunctional CD4+ and CD8+ T cells that were capable of manufacturing two or more cytokines simultaneously. Evidence of NKT cell proliferation and/or NKT cell-associated cytokine secretion was seen in most patients. In light of these strong responses, the concept of including NKT cell agonists in vaccine design requires further investigation.
Keywords: Dendritic cell; Melanoma; NKT cell; NY-ESO-1; α-Galactosylceramide.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

References
-
- Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. - DOI - PMC - PubMed
-
- Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, Ferrucci PF, Hill A, Wagstaff J, Carlino MS, Haanen JB, Maio M, Marquez-Rodas I, McArthur GA, Ascierto PA, Long GV, Callahan MK, Postow MA, Grossmann K, Sznol M, Dreno B, Bastholt L, Yang A, Rollin LM, Horak C, Hodi FS, Wolchok JD. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34. doi: 10.1056/NEJMoa1504030. - DOI - PMC - PubMed
-
- Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E, Savage KJ, Hernberg MM, Lebbe C, Charles J, Mihalcioiu C, Chiarion-Sileni V, Mauch C, Cognetti F, Arance A, Schmidt H, Schadendorf D, Gogas H, Lundgren-Eriksson L, Horak C, Sharkey B, Waxman IM, Atkinson V, Ascierto PA. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–330. doi: 10.1056/NEJMoa1412082. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

