Upstream kinases of plant SnRKs are involved in salt stress tolerance
- PMID: 29094495
- PMCID: PMC5814739
- DOI: 10.1111/tpj.13761
Upstream kinases of plant SnRKs are involved in salt stress tolerance
Abstract
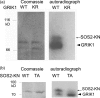
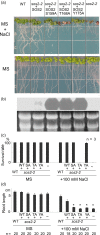
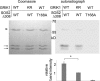
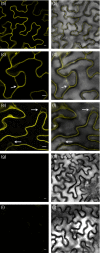
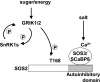
Sucrose non-fermenting 1-related protein kinases (SnRKs) are important for plant growth and stress responses. This family has three clades: SnRK1, SnRK2 and SnRK3. Although plant SnRKs are thought to be activated by upstream kinases, the overall mechanism remains obscure. Geminivirus Rep-Interacting Kinase (GRIK)1 and GRIK2 phosphorylate SnRK1s, which are involved in sugar/energy sensing, and the grik1-1 grik2-1 double mutant shows growth retardation under regular growth conditions. In this study, we established another Arabidopsis mutant line harbouring a different allele of gene GRIK1 (grik1-2 grik2-1) that grows similarly to the wild-type, enabling us to evaluate the function of GRIKs under stress conditions. In the grik1-2 grik2-1 double mutant, phosphorylation of SnRK1.1 was reduced, but not eliminated, suggesting that the grik1-2 mutation is a weak allele. In addition to high sensitivity to glucose, the grik1-2 grik2-1 mutant was sensitive to high salt, indicating that GRIKs are also involved in salinity signalling pathways. Salt Overly Sensitive (SOS)2, a member of the SnRK3 subfamily, is a critical mediator of the response to salinity. GRIK1 phosphorylated SOS2 in vitro, resulting in elevated kinase activity of SOS2. The salt tolerance of sos2 was restored to normal levels by wild-type SOS2, but not by a mutated form of SOS2 lacking the T168 residue phosphorylated by GRIK1. Activation of SOS2 by GRIK1 was also demonstrated in a reconstituted system in yeast. Our results indicate that GRIKs phosphorylate and activate SnRK1 and other members of the SnRK3 family, and that they play important roles in multiple signalling pathways in vivo.
Keywords: Arabidopsis thaliana; GRIKs; SOS2; SnRKs; phosphorylation; salinity; stress; sugar; upstream kinases.
© 2017 The Authors. The Plant Journal published by John Wiley & Sons Ltd and Society for Experimental Biology.
Figures



References
-
- Alonso, J.M. , Stepanova, A.N. , Leisse, T.J. et al (2003) Genome‐wide insertional mutagenesis of Arabidopsis thaliana . Science, 301, 653–657. - PubMed
-
- Baena‐González, E. , Rolland, F. , Thevelein, J.M. and Sheen, J. (2007) A central integrator of transcription networks in plant stress and energy signalling. Nature, 448, 938–942. - PubMed
-
- Bolle, C. , Huep, G. , Kleinbölting, N. , Haberer, G. , Mayer, K. , Leister, D. and Weisshaar, B. (2013) GABI‐DUPLO: a collection of double mutants to overcome genetic redundancy in Arabidopsis thaliana . Plant J. 75, 157–171. - PubMed
-
- Chaves‐Sanjuan, A. , Sanchez‐Barrena, M.J. , Gonzalez‐Rubio, J.M. , Moreno, M. , Ragel, P. , Jimenez, M. , Pardo, J.M. , Martinez‐Ripoll, M. , Quintero, F.J. and Albert, A. (2014) Structural basis of the regulatory mechanism of the plant CIPK family of protein kinases controlling ion homeostasis and abiotic stress. Proc. Natl Acad. Sci. USA, 111, E4532–E4541. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

