Strong responses of Drosophila melanogaster microbiota to developmental temperature
- PMID: 29095113
- PMCID: PMC5927714
- DOI: 10.1080/19336934.2017.1394558
Strong responses of Drosophila melanogaster microbiota to developmental temperature
Abstract
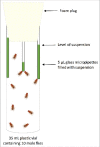
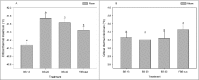
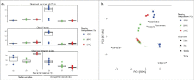
Physiological responses to changes in environmental conditions such as temperature may partly arise from the resident microbial community that integrates a wide range of bio-physiological aspects of the host. In the present study, we assessed the effect of developmental temperature on the thermal tolerance and microbial community of Drosophila melanogaster. We also developed a bacterial transplantation protocol in order to examine the possibility of reshaping the host bacterial composition and assessed its influence on the thermotolerance phenotype. We found that the temperature during development affected thermal tolerance and the microbial composition of male D. melanogaster. Flies that developed at low temperature (13°C) were the most cold resistant and showed the highest abundance of Wolbachia, while flies that developed at high temperature (31°C) were the most heat tolerant and had the highest abundance of Acetobacter. In addition, feeding newly eclosed flies with bacterial suspensions from intestines of flies developed at low temperatures changed the heat tolerance of recipient flies. However, we were not able to link this directly to a change in the host bacterial composition.
Keywords: Drosophila; climate change; developmental temperature; microbiota; thermal tolerance; transplantation.
Figures

References
-
- Donnelly A, Caffarra A, Kelleher CT, O'Neill BF, Diskin E, Pletsers A, Proctor H, Stirnemann R, O'Halloran J, Penuelas J, et al.. Surviving in a warmer world: environmental and genetic responses. Climate Research. 2012;53:245–262. doi: 10.3354/cr01102. - DOI
-
- Pacifici M, Foden WB, Visconti P, Watson JEM, Butchart SHM, Kovacs KM, Scheffers BR, Hole DG, Martin TG, Akçakaya HR, et al.. Assessing species vulnerability to climate change. Nature Climate Change. 2015;5:215–224. doi: 10.1038/nclimate2448. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
