Implementation of Rotavirus Surveillance and Vaccine Introduction - World Health Organization African Region, 2007-2016
- PMID: 29095805
- PMCID: PMC5689217
- DOI: 10.15585/mmwr.mm6643a7
Implementation of Rotavirus Surveillance and Vaccine Introduction - World Health Organization African Region, 2007-2016
Abstract
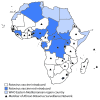
Rotavirus is a leading cause of severe pediatric diarrhea globally, estimated to have caused 120,000 deaths among children aged <5 years in sub-Saharan Africa in 2013 (1). In 2009, the World Health Organization (WHO) recommended rotavirus vaccination for all infants worldwide (2). Two rotavirus vaccines are currently licensed globally: the monovalent Rotarix vaccine (RV1, GlaxoSmithKline; 2-dose series) and the pentavalent RotaTeq vaccine (RV5, Merck; 3-dose series). This report describes progress of rotavirus vaccine introduction (3), coverage (using estimates from WHO and the United Nations Children's Fund [UNICEF]) (4), and impact on pediatric diarrhea hospitalizations in the WHO African Region. By December 2016, 31 (66%) of 47 countries in the WHO African Region had introduced rotavirus vaccine, including 26 that introduced RV1 and five that introduced RV5. Among these countries, rotavirus vaccination coverage (completed series) was 77%, according to WHO/UNICEF population-weighted estimates. In 12 countries with surveillance data available before and after vaccine introduction, the proportion of pediatric diarrhea hospitalizations that were rotavirus-positive declined 33%, from 39% preintroduction to 26% following rotavirus vaccine introduction. These results support introduction of rotavirus vaccine in the remaining countries in the region and continuation of rotavirus surveillance to monitor impact.
Conflict of interest statement
Figures
References
-
- Tate JE, Burton AH, Boschi-Pinto C, Parashar UD; World Health Organization–Coordinated Global Rotavirus Surveillance Network. Global, regional, and national estimates of rotavirus mortality in children <5 years of age, 2000–2013. Clin Infect Dis 2016;62(Suppl 2):S96–105. 10.1093/cid/civ1013 - DOI - PMC - PubMed
-
- World Health Organization. Rotavirus vaccines: an update. Wkly Epidemiol Rec 2009;84:533–40. - PubMed
-
- World Health Organization. Immunization vaccines and biologicals database, September 2016. Geneva, Switzerland: World Health Organization; 2016. http://www.who.int/immunization/monitoring_surveillance/data/en/
-
- World Health Organization (WHO). WHO/UNICEF estimates of national immunization coverage. Geneva, Switzerland: World Health Organization/United Nations Children’s Fund; 2016. http://www.who.int/immunization/monitoring_surveillance/routine/coverage...
-
- World Health Organization (WHO). WHO vaccine-preventable diseases: monitoring system. 2017 global summary. Geneva, Switzerland: World Health Organization; 2017. http://apps.who.int/immunization_monitoring/globalsummary/schedules
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical