Vaccination Coverage Among Children Aged 19-35 Months - United States, 2016
- PMID: 29095807
- PMCID: PMC5689213
- DOI: 10.15585/mmwr.mm6643a3
Vaccination Coverage Among Children Aged 19-35 Months - United States, 2016
Abstract
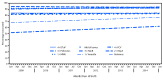
Vaccination is the most effective intervention to reduce morbidity and mortality from vaccine-preventable diseases in young children (1). Data from the 2016 National Immunization Survey-Child (NIS-Child) were used to assess coverage with recommended vaccines (2) among children aged 19-35 months in the United States. Coverage remained ≥90% for ≥3 doses of poliovirus vaccine (91.9%), ≥1 dose of measles, mumps, and rubella vaccine (MMR) (91.1%), ≥1 dose of varicella vaccine (90.6%), and ≥3 doses of hepatitis B vaccine (HepB) (90.5%). Coverage in 2016 was approximately 1-2 percentage points lower than in 2015 for ≥3 doses of diphtheria and tetanus toxoids and acellular pertussis vaccine (DTaP), ≥3 doses of poliovirus vaccine, the primary Haemophilus influenzae type b (Hib) series, ≥3 HepB doses, and ≥3 and ≥4 doses of pneumococcal conjugate vaccine (PCV), with no changes for other vaccines. More direct evaluation of trends by month and year of birth (3) found no change in coverage by age 2 years among children included in combined data from the 2015 and 2016 NIS-Child (born January 2012 through January 2015). The observed decreases in annual estimates might result from random differences in vaccination coverage by age 19 months between children sampled in 2016 and those sampled in 2015, among those birth cohorts eligible to be sampled in both survey years. For most vaccines, 2016 coverage was lower among non-Hispanic black* (black) children than among non-Hispanic white (white) children, and for children living below the federal poverty level† compared with those living at or above the poverty level. Vaccination coverage was generally lower among children insured by Medicaid (2.5-12.0 percentage points), and was much lower among uninsured children (12.4-24.9 percentage points), than among children with private insurance. The Vaccines for Children§ (VFC) program was designed to increase access to vaccines among children who might not otherwise be vaccinated because of inability to pay. Greater awareness and facilitating use of VFC might be helpful in reducing these disparities. Efforts should also be focused on minimizing breaks in continuity of health insurance and eliminating missed opportunities to vaccinate children during visits to health care providers. Despite the observed disparities and small changes in coverage from 2015, vaccination coverage among children aged 19-35 months remained high and stable in 2016.
Conflict of interest statement
Figures
References
-
- Robinson CL; Advisory Committee on Immunization Practices (ACIP), ACIP Child/Adolescent Immunization Work Group. Advisory Committee on Immunization Practices recommended immunization schedules for persons aged 0 through 18 years—United States, 2016. MMWR Morb Mortal Wkly Rep 2016;65:86–7. 10.15585/mmwr.mm6504a4 - DOI - PubMed
-
- CDC. Evaluating vaccination coverage trends with the National Immunization Survey-Child (NIS-Child), 2012–2016, United States. Atlanta, GA: US Department of Health and Human Services, CDC; 2017. https://www.cdc.gov/vaccines/imz-managers/coverage/childvaxview/pubs-pre...
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

