A core outcome set for adult cardiac surgery trials: A consensus study
- PMID: 29095881
- PMCID: PMC5667757
- DOI: 10.1371/journal.pone.0186772
A core outcome set for adult cardiac surgery trials: A consensus study
Abstract
Background: Invasive off- or on-pump cardiac surgery (elective and emergency procedures, excluding transplants are routinely performed to treat complications of ischaemic heart disease. Randomised controlled trials (RCT) evaluate the effectiveness of treatments in the setting of cardiac surgery. However, the impact of RCTs is weakened by heterogeneity in outcome measuring and reporting, which hinders comparison across trials. Core outcome sets (COS, a set of outcomes that should be measured and reported, as a minimum, in clinical trials for a specific clinical field) help reduce this problem. In light of the above, we developed a COS for cardiac surgery effectiveness trials.
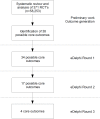
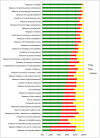
Methods: Potential core outcomes were identified a priori by analysing data on 371 RCTs of 58,253 patients. We reached consensus on core outcomes in an international three-round eDelphi exercise. Outcomes for which at least 60% of the participants chose the response option "no" and less than 20% chose the response option "yes" were excluded.
Results: Eighty-six participants from 23 different countries involving adult cardiac patients, cardiac surgeons, anaesthesiologists, nursing staff and researchers contributed to this eDelphi. The panel reached consensus on four core outcomes: 1) Measure of mortality, 2) Measure of quality of life, 3) Measure of hospitalisation and 4) Measure of cerebrovascular complication to be included in adult cardiac surgery trials.
Conclusion: This study used robust research methodology to develop a minimum core outcome set for clinical trials evaluating the effectiveness of treatments in the setting of cardiac surgery. As a next step, appropriate outcome measurement instruments have to be selected.
Conflict of interest statement
Figures
References
-
- American Heart Association. Heart Disease and Stroke Statistics—At-a-Glance. In: www.heart.org [Internet]. [cited 21 Jul 2015]. https://www.heart.org/idc/groups/ahamah-public/@wcm/@sop/@smd/documents/...
-
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. Lippincott Williams & Wilkins; 2015;131: e29–322. doi: 10.1161/CIR.0000000000000152 - DOI - PubMed
-
- World Health Organisation. ICTRP Search Portal Advanced Search [Internet]. 11 Sep 2016 pp. 1–2.
-
- Benstoem C, Moza A, Autschbach R, Stoppe C, Goetzenich A. Evaluating outcomes used in cardiothoracic surgery interventional research: a systematic review of reviews to develop a core outcome set. Benedetto U, editor. PLoS ONE. 2015;10: e0122204 doi: 10.1371/journal.pone.0122204 - DOI - PMC - PubMed
-
- Clarke M. Standardising outcomes for clinical trials and systematic reviews. Trials. 2007;8: 39 doi: 10.1186/1745-6215-8-39 - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials