Concurrent jellyfish blooms and tenacibaculosis outbreaks in Northern Norwegian Atlantic salmon (Salmo salar) farms
- PMID: 29095885
- PMCID: PMC5667831
- DOI: 10.1371/journal.pone.0187476
Concurrent jellyfish blooms and tenacibaculosis outbreaks in Northern Norwegian Atlantic salmon (Salmo salar) farms
Erratum in
-
Correction: Concurrent jellyfish blooms and tenacibaculosis outbreaks in Northern Norwegian Atlantic salmon (Salmo salar) farms.PLoS One. 2018 Jan 2;13(1):e0190762. doi: 10.1371/journal.pone.0190762. eCollection 2018. PLoS One. 2018. PMID: 29293696 Free PMC article.
Abstract
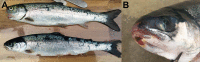
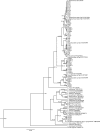
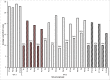
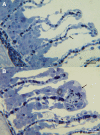
Tenacibaculosis is an increasing problem in the Norwegian Atlantic salmon aquaculture industry causing significant economic losses. In September 2015, two separate outbreaks of suspected tenacibaculosis occurred at two Atlantic salmon farms in Finnmark County in Northern Norway. The events resulted in major losses of smolts newly transferred into seawater. Prior to, and during the outbreaks, large numbers of small jellyfish, identified as Dipleurosoma typicum (Boeck) were observed in the vicinity of the farms and inside the net-pens. This study investigates the possible link between the jellyfish, Tenacibaculum spp. and the tenacibaculosis outbreaks. Bacteriology, histology, scanning and transmission electron microscopy, and real-time RT-PCR screening were performed on both fish and jellyfish samples. Based on the findings, Tenacibaculum finnmarkense was found to be the dominant bacteria associated with the tenacibaculosis outbreaks at both sites and that D. typicum is unlikely to be a vector for this fish pathogenic bacterium. However, results do show that the jellyfish caused direct damage to the fish's skin and may have exacerbated the bacterial infection by allowing an entry point for bacteria.
Conflict of interest statement
Figures







References
-
- Rutter T. Managing risk and uncertainty: OECD, Paris; 2010 [updated 15-16th April]. Available from: http://www.oecd.org/greengrowth/fisheries/45400772.pdf.
-
- Rodger HD, Henry L, Mitchell SO. Non-infectious gill disorders of marine salmonid fish. Reviews in Fish Biology and Fisheries. 2011;21(3):423–40. doi: 10.1007/s11160-010-9182-6 - DOI
-
- Boero F, Bouillon J, Gravili C, Miglietta MP, Parsons T, Piraino S. Gelatinous plankton: irregularities rule the world (sometimes). Marine Ecology Progress Series. 2008;356:299–310.
-
- Purcell JE, Uye S, Lo W. Anthropogenic causes of jellyfish blooms and their direct consequences for humans: a review. Marine Ecology Progress Series. 2007;350:153–74.
-
- Fleming NEC, Harrod C, Houghton JDR. Identifying potentially harmful jellyfish blooms using shoreline surveys. Aquaculture Environment Interactions. 2013;4:263–72.
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

