Comparison of the human gastric microbiota in hypochlorhydric states arising as a result of Helicobacter pylori-induced atrophic gastritis, autoimmune atrophic gastritis and proton pump inhibitor use
- PMID: 29095917
- PMCID: PMC5667734
- DOI: 10.1371/journal.ppat.1006653
Comparison of the human gastric microbiota in hypochlorhydric states arising as a result of Helicobacter pylori-induced atrophic gastritis, autoimmune atrophic gastritis and proton pump inhibitor use
Abstract
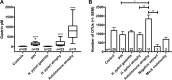
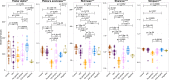
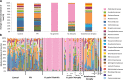
Several conditions associated with reduced gastric acid secretion confer an altered risk of developing a gastric malignancy. Helicobacter pylori-induced atrophic gastritis predisposes to gastric adenocarcinoma, autoimmune atrophic gastritis is a precursor of type I gastric neuroendocrine tumours, whereas proton pump inhibitor (PPI) use does not affect stomach cancer risk. We hypothesised that each of these conditions was associated with specific alterations in the gastric microbiota and that this influenced subsequent tumour risk. 95 patients (in groups representing normal stomach, PPI treated, H. pylori gastritis, H. pylori-induced atrophic gastritis and autoimmune atrophic gastritis) were selected from a cohort of 1400. RNA extracted from gastric corpus biopsies was analysed using 16S rRNA sequencing (MiSeq). Samples from normal stomachs and patients treated with PPIs demonstrated similarly high microbial diversity. Patients with autoimmune atrophic gastritis also exhibited relatively high microbial diversity, but with samples dominated by Streptococcus. H. pylori colonisation was associated with decreased microbial diversity and reduced complexity of co-occurrence networks. H. pylori-induced atrophic gastritis resulted in lower bacterial abundances and diversity, whereas autoimmune atrophic gastritis resulted in greater bacterial abundance and equally high diversity compared to normal stomachs. Pathway analysis suggested that glucose-6-phospahte1-dehydrogenase and D-lactate dehydrogenase were over represented in H. pylori-induced atrophic gastritis versus autoimmune atrophic gastritis, and that both these groups showed increases in fumarate reductase. Autoimmune and H. pylori-induced atrophic gastritis were associated with different gastric microbial profiles. PPI treated patients showed relatively few alterations in the gastric microbiota compared to healthy subjects.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer; 2013. Available from: http://globocan.iarc.fr/, accessed on 19/07/16.
-
- Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, et al. Helicobacter pylori infection and the development of gastric cancer. The New England journal of medicine. 2001;345(11):784–9. Epub 2001/09/15. doi: 10.1056/NEJMoa001999 . - DOI - PubMed
-
- Vannella L, Lahner E, Osborn J, Annibale B. Systematic review: gastric cancer incidence in pernicious anaemia. Aliment Pharmacol Ther. 2013;37(4):375–82. doi: 10.1111/apt.12177 . - DOI - PubMed
-
- Burkitt MD, Pritchard DM. Review article: Pathogenesis and management of gastric carcinoid tumours. Aliment Pharmacol Ther. 2006;24(9):1305–20. Epub 2006/10/25. doi: 10.1111/j.1365-2036.2006.03130.x . - DOI - PubMed
-
- Song H, Zhu J, Lu D. Long-term proton pump inhibitor (PPI) use and the development of gastric pre-malignant lesions. The Cochrane database of systematic reviews. 2014;(12):CD010623 Epub 2014/12/03. doi: 10.1002/14651858.CD010623.pub2 . - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

