Pyrazinamide clearance is impaired among HIV/tuberculosis patients with high levels of systemic immune activation
- PMID: 29095954
- PMCID: PMC5667771
- DOI: 10.1371/journal.pone.0187624
Pyrazinamide clearance is impaired among HIV/tuberculosis patients with high levels of systemic immune activation
Abstract
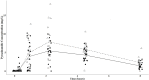
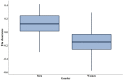
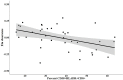
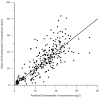
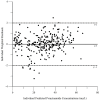
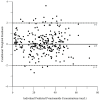
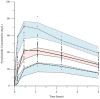
Pyrazinamide is the main driver of sterilizing effect in the standard regimen in adults and older children, and this effect is concentration-dependent. Tuberculosis patients co-infected with human immunodeficiency virus (HIV) have an increased risk for poor tuberculosis treatment outcomes and adverse drug events. We sought to determine whether measures of systemic immune activation were related to pyrazinamide pharmacokinetics among HIV/tuberculosis patients. We conducted a prospective cohort study of pyrazinamide pharmacokinetics in HIV/tuberculosis patients in Gaborone, Botswana. Patients underwent intensive pharmacokinetic sampling before and after the initiation of antiretroviral therapy, which can increase immune activation in HIV/tuberculosis. Compartmental pharmacokinetic modeling was performed to determine whether variability in systemic immune activation was related to variability in pyrazinamide pharmacokinetic parameters. Forty HIV/tuberculosis patients completed the first pharmacokinetic sampling visit, and 24 patients returned for a second visit following antiretroviral therapy initiation. The pyrazinamide plasma concentration-versus-time data were best explained by a one-compartment model with first-order elimination, and a combined additive and proportional residual error model. Pyrazinamide clearance was higher in men than women. Expression of CD38 and HLA- DR on CD8+T cells, a measure of HIV-associated immune activation, was inversely related to pyrazinamide clearance, with increasing immune activation associated with decreasing pyrazinamide clearance. Future studies should verify this finding in larger numbers of tuberculosis patients with and without HIV co-infection.
Conflict of interest statement
Figures


References
-
- Swaminathan S, Pasipanodya JG, Ramachandran G, Hemanth Kumar AK, Sristava S, Deshpande D, et al. Drug concentration thresholds predictive of therapy failure and death in children with tuberculosis: bread crumb trails in random forests. Clin Infect Dis. 2016;63(suppl 3):S63–74. doi: 10.1093/cid/ciw471 . - DOI - PMC - PubMed
-
- Chideya S, Winston CA, Peloquin CA, Bradford WZ, Hopewell PC, Wells CD, et al. Isoniazid, rifampin, ethambutol, and pyrazinamide pharmacokinetics and treatment outcomes among a predominantly HIV-infected cohort of adults with tuberculosis from Botswana. Clin Infect Dis. 2009;48(12):1685–94. doi: 10.1086/599040 . - DOI - PMC - PubMed
-
- Pasipanodya JG, McIlleron H, Burger A, Wash PA, Smith P, Gumbo T. Serum drug concentrations predictive of pulmonary tuberculosis outcomes. J Infect Dis. 2013;208(9):1464–73. doi: 10.1093/infdis/jit352 . - DOI - PMC - PubMed
-
- Chigutsa E, Pasipanodya JG, Visser ME, van Helden PD, Smith PJ, Sirgel FA, et al. Impact of nonlinear interactions of pharmacokinetics and MICs on sputum bacillary kill rates as a marker of sterilizing effect in tuberculosis. Antimicrob Agents Chemother. 2015;59(1):38–45. doi: 10.1128/AAC.03931-14 . - DOI - PMC - PubMed
-
- Gumbo T, Dona CS, Meek C, Leff R. Pharmacokinetics-pharmacodynamics of pyrazinamide in a novel in vitro model of tuberculosis for sterilizing effect: a paradigm for faster assessment of new antituberculosis drugs. Antimicrob Agents Chemother. 2009;53(8):3197–204. doi: 10.1128/AAC.01681-08 . - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

