Changes in extracellular matrix cause RPE cells to make basal deposits and activate the alternative complement pathway
- PMID: 29095988
- PMCID: PMC6251553
- DOI: 10.1093/hmg/ddx392
Changes in extracellular matrix cause RPE cells to make basal deposits and activate the alternative complement pathway
Abstract
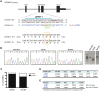
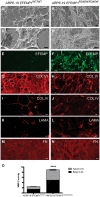
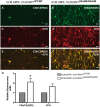
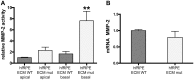
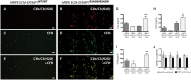
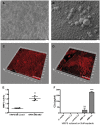
The design of efficient therapies for age-related macular degeneration (AMD) is limited by our understanding of the pathogenesis of basal deposits, which form between retinal pigment epithelium (RPE) and Bruch's membrane (BrM) early in disease, and involve activation of the complement system. To investigate the roles of BrM, RPE and complement in an AMD, we generated abnormal extracellular matrix (ECM) using CRISPR-edited ARPE-19 cells. We introduced to these cells the p.R345W mutation in EFEMP1, which causes early-onset macular degeneration. The abnormal ECM binds active complement C3 and causes the formation of basal deposits by normal human fetal (hf)RPE cells. Human fetal RPE (hfRPE) cells grown on abnormal ECM or BrM explants from AMD donors show chronic activation of the alternative complement pathway by excessive deposition of C3b. This process is exacerbated by impaired ECM turnover via increased matrix metalloproteinase-2 activity. The local cleavage of C3 via convertase-independent mechanisms can be a new therapeutic target for early AMD.
© The Author 2017. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures


References
-
- Miller J.W. (2013) Age-related macular degeneration revisited–piecing the puzzle: the LXIX Edward Jackson memorial lecture. Am. J. Ophthalmol., 155, 1–35.e13. - PubMed
-
- Evans J.R., Lawrenson J.G. (2012) Antioxidant vitamin and mineral supplements for slowing the progression of age-related macular degeneration. Cochrane Database Syst. Rev., 11, CD000254. - PubMed
-
- Newsome D.A., Huh W., Green W.R. (1987) Bruch's membrane age-related changes vary by region. Curr. Eye Res., 6, 1211–1221. - PubMed
-
- Curcio C.A., Johnson M. (2013) Structure, function, and pathology of Bruch's membrane. Retina, 1, 465–481.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

