Comparison of ferumoxytol-based cerebral blood volume estimates using quantitative R1 and R2* relaxometry
- PMID: 29096054
- PMCID: PMC5843511
- DOI: 10.1002/mrm.26975
Comparison of ferumoxytol-based cerebral blood volume estimates using quantitative R1 and R2* relaxometry
Abstract
Purpose: Cerebral perfusion is commonly assessed clinically with dynamic susceptibility contrast MRI using a bolus injection of gadolinium-based contrast agents, resulting in semi-quantitative values of cerebral blood volume (CBV). Steady-state imaging with ferumoxytol allows estimation of CBV with the potential for higher precision and accuracy. Prior CBV studies have focused on the signal disrupting T2* effects, but ferumoxytol also has high signal-enhancing T1 relaxivity. The purpose of this study was to investigate and compare CBV estimation using T1 and T2*, with the goal of understanding the contrast mechanisms and quantitative differences.
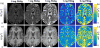
Methods: Changes in R1 (1/T1 ) and R2* (1/ T2*) were measured after the administration of ferumoxytol using high-resolution quantitative approaches. Images were acquired at 3.0T and R1 was estimated from an ultrashort echo time variable flip angle approach, while R2* was estimated from a multiple gradient echo sequence. Twenty healthy volunteers were imaged at two doses. CBV was derived and compared from relaxometry in gray and white matter using different approaches.
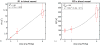
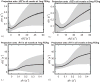
Results: R1 measurements showed a linear dependence of blood R1 with respect to dose in large vessels, in contrast to the nonlinear dose-dependence of blood R2* estimates. In the brain parenchyma, R2* showed linear dose-dependency whereas R1 showed nonlinearity. CBV calculations based on R2* changes in tissue and ferumoxytol blood concentration estimates based on R1 relaxivity showed the lowest variability in our cohort.
Conclusions: CBV measurements were successfully derived using a combined approach of R1 and R2* relaxometry. Magn Reson Med 79:3072-3081, 2018. © 2017 International Society for Magnetic Resonance in Medicine.
Keywords: R2*; R1; cerebral blood volume; ferumoxytol; gray matter; white matter.
© 2017 International Society for Magnetic Resonance in Medicine.
Figures


References
-
- Hachinski V, Iadecola C, Petersen RC, et al. National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke. 2006;37:2220–2241. - PubMed
-
- Roher AE. Cardiovascular system participation in Alzheimer’s disease pathogenesis. J Intern Med. 2015;277:426–428. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

