Chemical trapping and characterization of small oxoacids of sulfur (SOS) generated in aqueous oxidations of H2S
- PMID: 29096321
- PMCID: PMC5680521
- DOI: 10.1016/j.redox.2017.10.012
Chemical trapping and characterization of small oxoacids of sulfur (SOS) generated in aqueous oxidations of H2S
Abstract
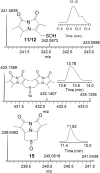
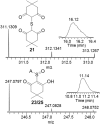
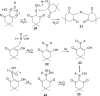
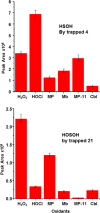
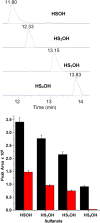
Small oxoacids of sulfur (SOS) are elusive molecules like sulfenic acid, HSOH, and sulfinic acid, HS(O)OH, generated during the oxidation of hydrogen sulfide, H2S, in aqueous solution. Unlike their alkyl homologs, there is a little data on their generation and speciation during H2S oxidation. These SOS may exhibit both nucleophilic and electrophilic reactivity, which we attribute to interconversion between S(II) and S(IV) tautomers. We find that SOS may be trapped in situ by derivatization with nucleophilic and electrophilic trapping agents and then characterized by high resolution LC MS. In this report, we compare SOS formation from H2S oxidation by a variety of biologically relevant oxidants. These SOS appear relatively long lived in aqueous solution, and thus may be involved in the observed physiological effects of H2S.
Keywords: And bromobimane; Dimedone; Hydrogen sulfide; Sulfenic acid; Sulfinic acid.
Copyright © 2017 The Authors. Published by Elsevier B.V. All rights reserved.
Figures

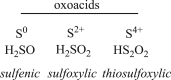







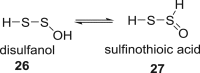



References
-
- Szabó C. Hydrogen sulphide and its therapeutic potential. Nat. Rev. Drug Discov. 2007;6:917–935. - PubMed
-
- Wang R. Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiol. Rev. 2012;92:791–896. - PubMed
-
- Hosoki R., Matsuki N., Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem. Biophys. Res. Commun. 1997;237:527–531. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

