Glucocorticosteroids for people with alcoholic hepatitis
- PMID: 29096421
- PMCID: PMC6491283
- DOI: 10.1002/14651858.CD001511.pub3
Glucocorticosteroids for people with alcoholic hepatitis
Update in
-
Glucocorticosteroids for people with alcoholic hepatitis.Cochrane Database Syst Rev. 2019 Apr 9;4(4):CD001511. doi: 10.1002/14651858.CD001511.pub4. Cochrane Database Syst Rev. 2019. PMID: 30964545 Free PMC article.
Abstract
Background: Alcoholic hepatitis is a form of alcoholic liver disease, characterised by steatosis, necroinflammation, fibrosis, and potential complications to the liver disease. Typically, alcoholic hepatitis presents in people between 40 and 50 years of age. Alcoholic hepatitis can be resolved if people abstain from drinking, but the risk of death will depend on the severity of the liver damage and abstinence from alcohol. Glucocorticosteroids are used as anti-inflammatory drugs for people with alcoholic hepatitis. Glucocorticosteroids have been studied extensively in randomised clinical trials in order to assess their benefits and harms. However, the results have been contradictory.
Objectives: To assess the benefits and harms of glucocorticosteroids in people with alcoholic hepatitis.
Search methods: We identified trials through electronic searches in Cochrane Hepato-Biliary's (CHB) Controlled Trials Register, CENTRAL, MEDLINE, Embase, LILACS, and Science Citation Index Expanded. We looked for ongoing or unpublished trials in clinical trials registers and pharmaceutical company sources. We also scanned reference lists of the studies retrieved. The last search was 20 October 2016.
Selection criteria: Randomised clinical trials assessing glucocorticosteroids versus placebo or no intervention in people with alcoholic hepatitis, irrespective of year, language of publication, or format. We considered trials with adult participants diagnosed with alcoholic hepatitis, which could have been established through clinical or biochemical diagnostic criteria or both. We defined alcoholic hepatitis as mild (Maddrey's score less than 32) and severe (Maddrey's score 32 or more). We allowed co-interventions in the trial groups, provided they were similar.
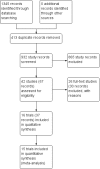
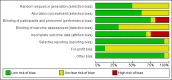
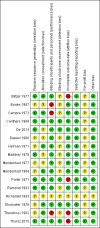
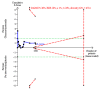
Data collection and analysis: We followed Cochrane and CHB methodology, performing the meta-analyses using Review Manager 5 and Trial Sequential Analysis. We presented the results of dichotomous outcomes as risk ratios (RR) and those of the continuous outcomes as mean difference (MD). We applied both the fixed-effect model and the random-effects model meta-analyses. Whenever there were significant discrepancies in the results, we reported the more conservative point estimate of the two. We considered a P value of 0.01 or less, two-tailed, as statistically significant if the required information size was reached due to our three primary outcomes (all-cause mortality, health-related quality of life, and serious adverse events during treatment) and our post hoc decision to include analyses of mortality at more time points. We presented heterogeneity using the I² statistic. If trialists used intention-to-treat analysis to deal with missing data, we used these data in our primary analysis; otherwise, we used the available data. We assessed the bias risk of the trials using bias risk domains and the quality of the evidence using GRADE.
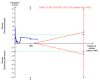
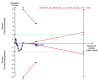
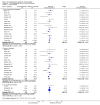
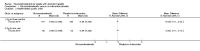
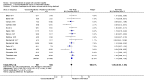
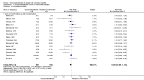
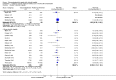
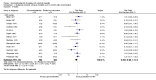
Main results: Sixteen trials fulfilled the inclusion criteria. All trials were at high risk of bias. Fifteen trials provided data for analysis (927 participants received glucocorticosteroids and 934 participants received placebo or no intervention). The glucocorticosteroids were administered orally or parenterally for a median of 28 days (range 3 days to 12 weeks). The participants were between 25 and 70 years old, had different stages of alcoholic liver disease, and 65% were men. The follow-up of trial participants, when it was reported, was up to the moment of discharge from the hospital, until they died (a median of 63 days), or for at least a year. There was no evidence of effect of glucocorticosteroids on all-cause mortality up to three months following randomisation neither with traditional meta-analysis (random-effects RR 0.90, 95% CI 0.70 to 1.15; participants = 1861; trials = 15; I² = 45% (moderate heterogeneity) nor with Trial Sequential Analysis. Meta-analysis showed no evidence of effect on health-related quality of life up to three months (MD -0.04 points; 95% CI -0.11 to 0.03; participants = 377; trial = 1; low-quality evidence), measured with the European Quality of Life - 5 Dimensions-3 Levels (EQ- 5D-3L) scale. There was no evidence of effect on the occurrence of serious adverse events during treatment, neither with traditional meta-analysis (random-effects RR 1.05, 95% CI 0.85 to 1.29; participants = 1861; trials = 15; I² = 36% (moderate heterogeneity), liver-related mortality up to three months following randomisation (random-effects RR 0.89, 95% CI 0.69 to 1.14; participants = 1861; trials = 15; I² = 46% (moderate heterogeneity), frequency of any complications up to three months following randomisation (random-effects RR 1.04, 95% CI 0.86 to 1.27; participants = 1861; I² = 42% (moderate heterogeneity), and frequency of non-serious adverse events up to three months' follow-up after end of treatment (random-effects RR 1.99, 95% CI 0.72 to 5.48; participants = 160; trials = 4; I² = 0% (no heterogeneity) nor with Trial Sequential Analysis. Nine of the trials were industry-funded.
Authors' conclusions: We found no evidence of a difference between glucocorticosteroids and placebo or no intervention on all-cause mortality, health-related quality of life, and serious adverse events during treatment. The risk of bias was high and the quality of evidence was very low or low. Therefore, we are very uncertain about this effect estimate. Due to inadequate reporting, we cannot exclude increases in adverse events. As the confidence intervals were wide, we cannot rule out significant benefits and harms of glucocorticosteroids. Therefore, we need placebo-controlled, randomised clinical trials, designed according to the SPIRIT guidelines and reported according to the CONSORT guidelines. Future trials ought to report depersonalised individual participant data, so that proper individual participant data meta-analyses of the effects of glucocorticosteroids in subgroups can be conducted.
Conflict of interest statement
CP: no financial, academic, or personal conflicts of interest. DV: no financial, academic, or personal conflicts of interest. GC: no financial, academic, or personal conflicts of interest. ET: no financial, academic, or personal conflicts of interest. DN: no financial, academic, or personal conflicts of interest. CG: no financial, academic, or personal conflicts of interest.
Figures
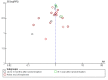
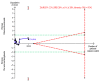
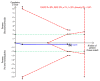
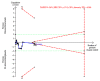






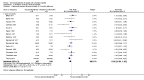

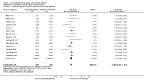

References
References to studies included in this review
-
- Blitzer BL, Mutchnick MG, Joshi PH, Phillips MM, Fessel JM, Conn HO. Adrenocorticosteroid therapy in alcoholic hepatitis. A prospective, double‐blind randomized study. Digestive Diseases 1977;22(6):477‐84. [MEDLINE: ] - PubMed
- Blitzer BL, Mutchnick MG, Joshi PH, Phillips MM, Fessel JM, Conn HO. Adrenocorticosteroid therapy in alcoholic hepatitis. A prospective, double‐blind randomized study. Gastroenterology 1973;64:880. - PubMed
-
- Bories P, Guedj JY, Mirouze D, Yousfi A, Michel H. Prednisolone treatment of alcoholic hepatitis: forty‐five patients [Traitement de l'hepatite alcoolique aigue par la prednisolone. Quarante‐cinq malades]. La Presse Medicale 1987;16:769‐72. [MEDLINE: ] - PubMed
-
- Campra JL, Hamlin EM Jr, Kirshbaum RJ, Olivier M, Redeker AG, Reynolds TB. Prednisone therapy of acute alcoholic hepatitis. Report of a controlled trial. Annals of Internal Medicine 1973;79(5):625‐31. [MEDLINE: ] - PubMed
-
- Erratum: Methylprednisolone therapy in patients with severe alcoholic hepatitis: Randomized multicenter trial (American Journal of Gastroenterology, Vol. 85, No. 4 (473)). American Journal of Gastroenterology1990; Vol. 85, issue 6:776. [CRS: 6800131000045704; EMBASE: 1990185566]
- Carithers RL Jr, Herlong HF, Diehl AM, Shaw EW, Combes B, Fallon HJ, et al. Methylprednisolone therapy in patients with severe alcoholic hepatitis. A randomized multicenter trial. Annals of Internal Medicine 1989;110:685‐90. [MEDLINE: ] - PubMed
- Carithers RLJ, Herlong HF, Diehl AM, Shaw EW, Combes B, Fallon HJ, et al. Corticosteroid therapy of alcoholic hepatitis: how many studies will it take?. Hepatology (Baltimore, Md.) 1990;12(3):619‐20. [CENTRAL: 844269; CRS: 6800100000024896; CN‐00844269] - PubMed
- Maddrey WC, Carithers R Jr, Herlong HF, Combes B, Diehl AM, Shaw E, et al. Prednisolone therapy in patients with severe alcoholic hepatitis: results of a multicenter trial. Hepatology (Baltimore, Md.) 1986;6(5):1202.
References to studies excluded from this review
-
- Alvarez MA, Cabre E, Lorenzo‐Zuniga V, Montoliu S, Planas R, Gassull MA. Combining steroids with enteral nutrition: a better therapeutic strategy for severe alcoholic hepatitis? Results of a pilot study. European Journal of Gastroenterology & Hepatology 2004;16:1375‐80. - PubMed
-
- Cabré E, Rodríguez‐Iglesias P, Caballería J, Quer JC, Sánchez‐Lombraña JL, Parés A, et al. Short‐ and long‐term outcome of severe alcohol‐induced hepatitis treated with steroids or enteral nutrition: a multicenter randomized trial. Hepatology (Baltimore, Md.) 2000;32:36‐42. - PubMed
- Cabré E, on behalf of the Spanish Group for the Study of Alcoholic Hepatitis. Treatment of severe alcoholic hepatitis with steroids or total enteral nutrition: interim results of a prospective, randomized, multicentric trial. Gastroenterology 1998;114:A868‐9.
- Gassull M, Cabré E. Short and long‐term outcome in severe alcoholic hepatitis (SAH) treated with steroids or total enteral nutrition (TEN). A multicentric randomized controlled trial by the Spanish Group for the Study of Alcoholic Hepatitis. Journal of Parenteral and Enteral Nutrition 2000;24:S5.
-
- Christensen E, Fauerholdt L, Schlichting P, Juhl E, Poulsen H, Tygstrup N. Aspects of the natural history of gastrointestinal bleeding in cirrhosis and the effect of prednisone. Gastroenterology 1981;81(5):944‐52. - PubMed
-
- Copenhagen Study Group for Liver Diseases. Effect of prednisone on the survival of patients with cirrhosis of the liver. A report from the Copenhagen Study Group for Liver Diseases. Lancet 1969;1(586):119‐21. [CENTRAL: 844264; CRS: 6800100000024872; PUBMED: 69076529] - PubMed
-
- Daures J‐P, Peray P, Bories P, Blanc P, Yousfi A, Michel H, et al. Steroid therapy in acute alcoholic hepatitis: a pooled estimate based on published randomized controlled trials [Place de la corticotherapie dans le traitement des hépatites alcooliques aiguës. Résultants d'une méta‐analyse]. Gastroenterologie Clinique et Biologique 1991;51:223‐8. - PubMed
Additional references
-
- O'Shea RS, Dasarathy S, McCullough AJ. Alcoholic liver disease. Hepatology (Baltimore, Md.) 2010;51:307‐28. - PubMed
-
- Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401‐6. [PUBMED: 21208779] - PubMed
-
- Becker U, Deis A, Sorensen TI, et al. Prediction of risk of liver disease by alcohol intake, sex, and age: a prospective population study. Hepatology (Baltimore, Md.) 1996;23:1025‐9. - PubMed
-
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50(4):1088‐101. [PUBMED: 7786990] - PubMed
References to other published versions of this review
-
- Saconato H, Gluud C, Christensen E, Atallah ÁN. Glucocorticosteroids for alcoholic hepatitis. Cochrane Database of Systematic Reviews 1999, Issue 1. [DOI: 10.1002/14651858.CD001511] - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

