Therapy response testing of breast cancer in a 3D high-throughput perfused microfluidic platform
- PMID: 29096610
- PMCID: PMC5668957
- DOI: 10.1186/s12885-017-3709-3
Therapy response testing of breast cancer in a 3D high-throughput perfused microfluidic platform
Abstract
Background: Breast cancer is the most common invasive cancer among women. Currently, there are only a few models used for therapy selection, and they are often poor predictors of therapeutic response or take months to set up and assay. In this report, we introduce a microfluidic OrganoPlate® platform for extracellular matrix (ECM) embedded tumor culture under perfusion as an initial study designed to investigate the feasibility of adapting this technology for therapy selection.
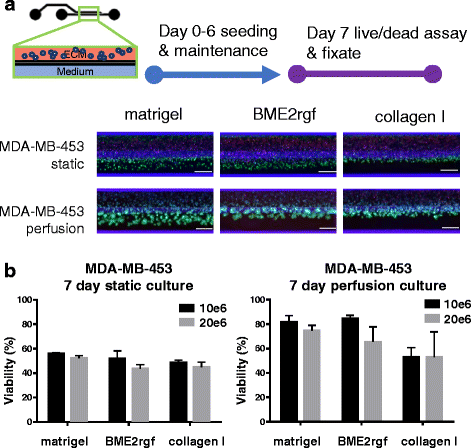
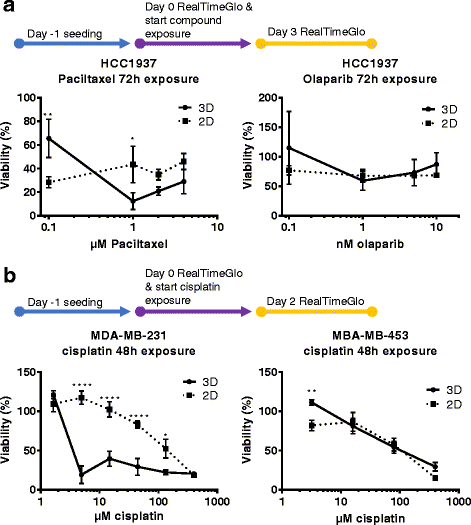
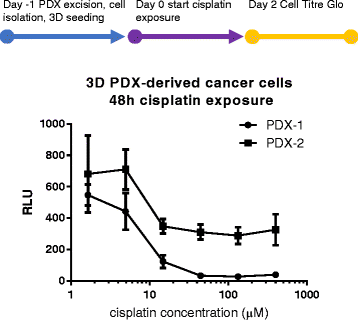
Methods: The triple negative breast cancer cell lines MDA-MB-453, MDA-MB-231 and HCC1937 were selected based on their different BRCA1 and P53 status, and were seeded in the platform. We evaluate seeding densities, ECM composition (Matrigel®, BME2rgf, collagen I) and biomechanical (perfusion vs static) conditions. We then exposed the cells to a series of anti-cancer drugs (paclitaxel, olaparib, cisplatin) and compared their responses to those in 2D cultures. Finally, we generated cisplatin dose responses in 3D cultures of breast cancer cells derived from 2 PDX models.
Results: The microfluidic platform allows the simultaneous culture of 96 perfused micro tissues, using limited amounts of material, enabling drug screening of patient-derived material. 3D cell culture viability is improved by constant perfusion of the medium. Furthermore, the drug response of these triple negative breast cancer cells was attenuated by culture in 3D and differed from that observed in 2D substrates.
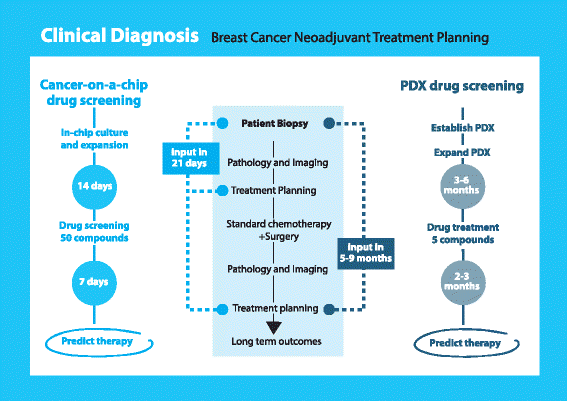
Conclusions: We have investigated the use of a high-throughput organ-on-a-chip platform to select therapies. Our results have raised the possibility to use this technology in personalized medicine to support selection of appropriate drugs and to predict response to therapy in a real time fashion.
Keywords: Organ-on-a-chip; P53 and BRCA1; Personalized medicine; Triple negative.
Conflict of interest statement
Ethics approval and consent to participate
The generation of the breast cancer PDX models were described previously [37]. The Mayo Clinic Institutional Animal Care and Use Committee (IACUC) reviewed and approved all of the mouse experiments for the PDX tumors used in this study.
Consent for publication
Not applicable
Competing interests
Paul Vulto, Jos Joore, Thomas Hankemeier and Sebastiaan J Trietsch have ownership interest in Mimetas B.V, which has developed the technology reported in this publication. Henriette Lanz, Anthony Saleh, Bart Kramer and Chee Ping Ng are employees of Mimetas B.V.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

