Measuring what matters to rare disease patients - reflections on the work by the IRDiRC taskforce on patient-centered outcome measures
- PMID: 29096663
- PMCID: PMC5667521
- DOI: 10.1186/s13023-017-0718-x
Measuring what matters to rare disease patients - reflections on the work by the IRDiRC taskforce on patient-centered outcome measures
Abstract
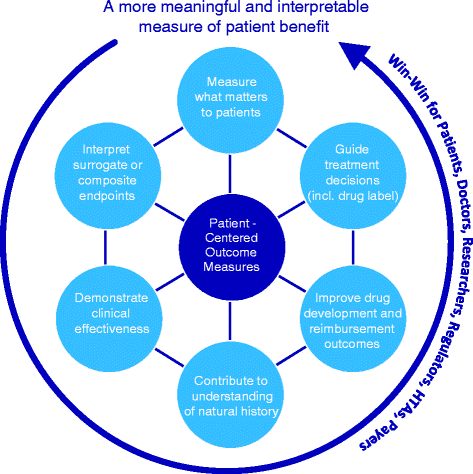
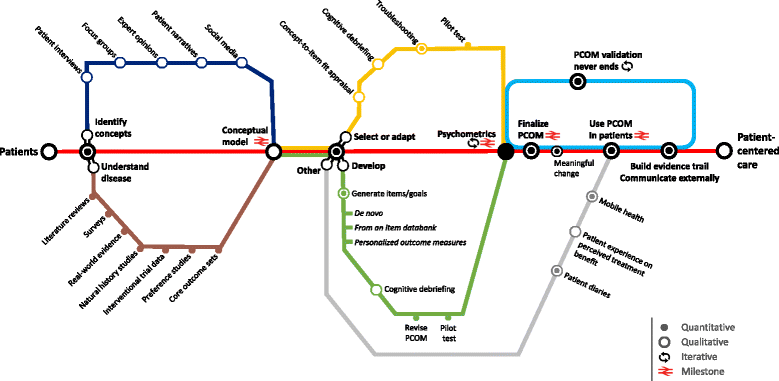
Our ability to evaluate outcomes which genuinely reflect patients' unmet needs, hopes and concerns is of pivotal importance. However, much current clinical research and practice falls short of this objective by selecting outcome measures which do not capture patient value to the fullest. In this Opinion, we discuss Patient-Centered Outcomes Measures (PCOMs), which have the potential to systematically incorporate patient perspectives to measure those outcomes that matter most to patients. We argue for greater multi-stakeholder collaboration to develop PCOMs, with rare disease patients and families at the center. Beyond advancing the science of patient input, PCOMs are powerful tools to translate care or observed treatment benefit into an 'interpretable' measure of patient benefit, and thereby help demonstrate clinical effectiveness. We propose mixed methods psychometric research as the best route to deliver fit-for-purpose PCOMs in rare diseases, as this methodology brings together qualitative and quantitative research methods in tandem with the explicit aim to efficiently utilise data from small samples. And, whether one opts to develop a brand-new PCOM or to select or adapt an existing outcome measure for use in a rare disease, the anchors remain the same: patients, their daily experience of the rare disease, their preferences, core concepts and values. Ultimately, existing value frameworks, registries, and outcomes-based contracts largely fall short of consistently measuring the full range of outcomes that matter to patients. We argue that greater use of PCOMs in rare diseases would enable a fast track to Patient-Centered Care.
Keywords: Clinical outcome assessments; Mixed methods research; Patient centricity; Patient-centered outcome measures; Patient-focused drug development (PFDD); Patient-relevant outcomes; Patient-reported outcomes; Rare diseases; Rasch measurement theory.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Figures
References
-
- European Commission Inventory of Union and Member State incentives to support research into, and the development and avalaibility of, orphan medicinal products - state of play 2015: European Commission; 2016.
-
- US Food and Drug Administration (FDA). The Rise in Orphan Drug Designations: Meeting the Growing Demand. FDA Voice, 18/07/2016. http://blogs.fda.gov/fdavoice/index.php/2016/07/the-rise-in-orphan-drug-.... Accessed 01 June 2017.
-
- Cortelis Regulatory Intelligence. Clinical trial data seen as inadequate to support approval for Sarepta's DMD treatment. AdComm Bulletin, 2016.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

