HIV treatment simplification to elvitegravir/cobicistat/emtricitabine/tenofovir disproxil fumarate (E/C/F/TDF) plus darunavir: a pharmacokinetic study
- PMID: 29096670
- PMCID: PMC5669010
- DOI: 10.1186/s12981-017-0185-4
HIV treatment simplification to elvitegravir/cobicistat/emtricitabine/tenofovir disproxil fumarate (E/C/F/TDF) plus darunavir: a pharmacokinetic study
Abstract
Background: As a simplification strategy for treatment-experienced HIV-infected patients who have achieved virologic suppression on a multi-drug, multi-class antiretroviral regimen, the aim of this study was to evaluate the safety, efficacy, and pharmacokinetics of once-daily elvitegravir/cobicistat/emtricitabine/tenofovir disproxil fumarate (E/C/F/TDF) with darunavir.
Methods: A single arm, open-label 48-week study was conducted of regimen simplification to E/C/F/TDF plus darunavir 800 mg daily from stable therapy including two nucleoside/nucleotide reverse transcriptase inhibitors, a ritonavir-boosted protease inhibitor, and an integrase inhibitor. Participants had plasma HIV viral load consistently < 200 copies/mL for ≥ 6 months, estimated glomerular filtration rate (eGFR) ≥ 60 mL/min, and no genotypic resistance to major components of the study regimen. Plasma viral load was measured at weeks 2 and 4, then every 4 weeks throughout the study. Safety laboratory assessments were conducted at baseline and at weeks 12, 24, 36, and 48. Antiretroviral drug concentrations were measured at baseline and once ≥ 2 weeks after the regimen change.
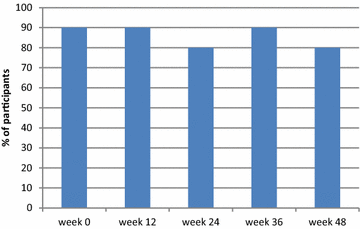
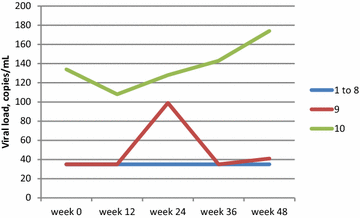
Results: Ten HIV-infected adults (8 male and 2 female; median age 50.5 years) were enrolled. All maintained virologic suppression on the new regimen for 48 weeks. One subject experienced a decrease in eGFR from 62 mL/min at baseline to 52 mL/min at week 12; study medications were continued and his eGFR remained stable (50-59 mL/min) thereafter. No subjects discontinued study medications for renal function changes or other adverse events. Darunavir trough concentration were lower on the new regimen than on darunavir/ritonavir 800/100 mg (n = 5; p < 0.05).
Conclusions: Despite low darunavir trough concentrations, treatment simplification to a two-pill, once-daily regimen of E/C/F/TDF plus darunavir was safe and effective for 48 weeks among 10 selected treatment-experienced HIV-infected patients. Trial registration The study protocol was registered with ClinicalTrials.gov (NCT02199613) on July 22, 2014.
Keywords: Antiretrovirals; Cobicistat; Darunavir; Elvitegravir; HIV.
Figures
Similar articles
-
Simplification to coformulated elvitegravir, cobicistat, emtricitabine, and tenofovir versus continuation of ritonavir-boosted protease inhibitor with emtricitabine and tenofovir in adults with virologically suppressed HIV (STRATEGY-PI): 48 week results of a randomised, open-label, phase 3b, non-inferiority trial.Lancet Infect Dis. 2014 Jul;14(7):581-9. doi: 10.1016/S1473-3099(14)70782-0. Epub 2014 Jun 5. Lancet Infect Dis. 2014. PMID: 24908551 Clinical Trial.
-
Safety, efficacy, and pharmacokinetics of a single-tablet regimen containing elvitegravir, cobicistat, emtricitabine, and tenofovir alafenamide in treatment-naive, HIV-infected adolescents: a single-arm, open-label trial.Lancet HIV. 2016 Dec;3(12):e561-e568. doi: 10.1016/S2352-3018(16)30121-7. Epub 2016 Oct 17. Lancet HIV. 2016. PMID: 27765666 Clinical Trial.
-
Week 96 efficacy and safety results of the phase 3, randomized EMERALD trial to evaluate switching from boosted-protease inhibitors plus emtricitabine/tenofovir disoproxil fumarate regimens to the once daily, single-tablet regimen of darunavir/cobicistat/emtricitabine/tenofovir alafenamide (D/C/F/TAF) in treatment-experienced, virologically-suppressed adults living with HIV-1.Antiviral Res. 2019 Oct;170:104543. doi: 10.1016/j.antiviral.2019.104543. Epub 2019 Jul 4. Antiviral Res. 2019. PMID: 31279073 Clinical Trial.
-
Darunavir/Cobicistat/Emtricitabine/Tenofovir Alafenamide: A Review in HIV-1 Infection.Drugs. 2018 Jul;78(10):1013-1024. doi: 10.1007/s40265-018-0934-2. Drugs. 2018. PMID: 29915897 Review.
-
Elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate single-tablet regimen (Stribild®): a review of its use in the management of HIV-1 infection in adults.Drugs. 2014 Jan;74(1):75-97. doi: 10.1007/s40265-013-0158-4. Drugs. 2014. PMID: 24338165 Review.
Cited by
-
Effectiveness of the combination elvitegravir/cobicistat/tenofovir/emtricitabine (EVG/COB/TFV/FTC) plus darunavir among treatment-experienced patients in clinical practice: a multicentre cohort study.AIDS Res Ther. 2020 Jul 20;17(1):45. doi: 10.1186/s12981-020-00302-2. AIDS Res Ther. 2020. PMID: 32690099 Free PMC article.
References
-
- Harris M, Harrigan PR, Montaner JSG. Chapter 46: Antiretroviral Therapy of Drug-resistant HIV. In: Volberding PA, Sande MA, Lange J, Greene WC, Gallant JE, editors. Global HIV/AIDS medicine. Saunders: Elsevier; 2008. pp. 537–545.
-
- Yazdanpanah Y, Fagard C, Descamps D, et al. High rate of virologic suppression with raltegravir plus etravirine and darunavir/ritonavir among treatment-experienced patients infected with multi-drug resistant HIV: results of the ANRS 139 TRIO trial. Clin Infect Dis. 2009;49:1441–1449. doi: 10.1086/630210. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous