Signal Dynamics and Interactions during Flooding Stress
- PMID: 29097391
- PMCID: PMC5813540
- DOI: 10.1104/pp.17.01232
Signal Dynamics and Interactions during Flooding Stress
Abstract
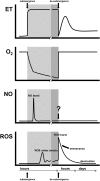
Flooding is detrimental for nearly all higher plants, including crops. The compound stress elicited by slow gas exchange and low light levels under water is responsible for both a carbon and an energy crisis ultimately leading to plant death. The endogenous concentrations of four gaseous compounds, oxygen, carbon dioxide, ethylene, and nitric oxide, change during the submergence of plant organs in water. These gases play a pivotal role in signal transduction cascades, leading to adaptive processes such as metabolic adjustments and anatomical features. Of these gases, ethylene is seen as the most consistent, pervasive, and reliable signal of early flooding stress, most likely in tight interaction with the other gases. The production of reactive oxygen species (ROS) in plant cells during flooding and directly after subsidence, during which the plant is confronted with high light and oxygen levels, is characteristic for this abiotic stress. Low, well-controlled levels of ROS are essential for adaptive signaling pathways, in interaction with the other gaseous flooding signals. On the other hand, excessive uncontrolled bursts of ROS can be highly damaging for plants. Therefore, a fine-tuned balance is important, with a major role for ROS production and scavenging. Our understanding of the temporal dynamics of the four gases and ROS is basal, whereas it is likely that they form a signature readout of prevailing flooding conditions and subsequent adaptive responses.
© 2018 American Society of Plant Biologists. All Rights Reserved.
Figures



References
-
- Akman M, Kleine R, van Tienderen PH, Schranz ME (2017) Identification of the submergence tolerance QTL Come Quick Drowning1 (CQD1) in Arabidopsis thaliana. J Hered 108: 308–317 - PubMed
-
- Alpuerto JB, Hussain RM, Fukao T (2016) The key regulator of submergence tolerance, SUB1A, promotes photosynthetic and metabolic recovery from submergence damage in rice leaves. Plant Cell Environ 39: 672–684 - PubMed
-
- Asgher M, Per TS, Masood A, Fatma M, Freschi L, Corpas FJ, Khan NA (2017) Nitric oxide signaling and its crosstalk with other plant growth regulators in plant responses to abiotic stress. Environ Sci Pollut Res Int 24: 2273–2285 - PubMed
-
- Astier J, Rasul S, Koen E, Manzoor H, Besson-Bard A, Lamotte O, Jeandroz S, Durner J, Lindermayr C, Wendehenne D (2011) S-Nitrosylation: an emerging post-translational protein modification in plants. Plant Sci 181: 527–533 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

