Antibody-dependent enhancement of severe dengue disease in humans
- PMID: 29097492
- PMCID: PMC5858873
- DOI: 10.1126/science.aan6836
Antibody-dependent enhancement of severe dengue disease in humans
Abstract
For dengue viruses 1 to 4 (DENV1-4), a specific range of antibody titer has been shown to enhance viral replication in vitro and severe disease in animal models. Although suspected, such antibody-dependent enhancement of severe disease has not been shown to occur in humans. Using multiple statistical approaches to study a long-term pediatric cohort in Nicaragua, we show that risk of severe dengue disease is highest within a narrow range of preexisting anti-DENV antibody titers. By contrast, we observe protection from all symptomatic dengue disease at high antibody titers. Thus, immune correlates of severe dengue must be evaluated separately from correlates of protection against symptomatic disease. These results have implications for studies of dengue pathogenesis and for vaccine development, because enhancement, not just lack of protection, is of concern.
Copyright © 2017, American Association for the Advancement of Science.
Figures
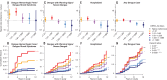

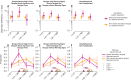

Comment in
-
Advancing dengue vaccine development.Science. 2017 Nov 17;358(6365):865-866. doi: 10.1126/science.aaq0215. Science. 2017. PMID: 29146795 No abstract available.
References
-
- World Health Organization (WHO)/Special Programme for Research and Training in Tropical Diseases (TDR) , “Dengue: Guidelines for diagnosis, treatment, prevention and control” (WHO, 2009).
-
- WHO , “Dengue haemorrhagic fever: Diagnosis, treatment, prevention and control” (WHO, ed. 2, 1997).
-
- Sangkawibha N, et al. , Am. J. Epidemiol. 120, 653–669 (1984). - PubMed
-
- Guzman M. G, Harris E, Lancet 385, 453–465 (2015). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

