DnaJ Heat Shock Protein Family B Member 9 Is a Novel Biomarker for Fibrillary GN
- PMID: 29097623
- PMCID: PMC5748911
- DOI: 10.1681/ASN.2017030306
DnaJ Heat Shock Protein Family B Member 9 Is a Novel Biomarker for Fibrillary GN
Abstract
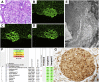
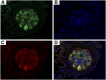
Fibrillary GN (FGN) is a rare primary glomerular disease. Histologic and histochemical features of FGN overlap with those of other glomerular diseases, and no unique histologic biomarkers for diagnosing FGN have been identified. We analyzed the proteomic content of glomeruli in patient biopsy specimens and detected DnaJ heat shock protein family (Hsp40) member B9 (DNAJB9) as the fourth most abundant protein in FGN glomeruli. Compared with amyloidosis glomeruli, FGN glomeruli exhibited a >6-fold overexpression of DNAJB9 protein. Sanger sequencing and protein sequence coverage maps showed that the DNAJB9 protein deposited in FGN glomeruli did not have any major sequence or structural alterations. Notably, we detected DNAJB9 in all patients with FGN but not in healthy glomeruli or in 19 types of non-FGN glomerular diseases. We also observed the codeposition of DNAJB9 and Ig-γ Overall, these findings indicate that DNAJB9 is an FGN marker with 100% sensitivity and 100% specificity. The magnitude and specificity of DNAJB9 overabundance in FGN also suggests that this protein has a role in FGN pathogenesis. With this evidence, we propose that DNAJB9 is a strong biomarker for rapid diagnosis of FGN in renal biopsy specimens.
Keywords: DNAJB9; biomarker; fibrillary GN; immunofluorescence; proteomics; renal.
Copyright © 2018 by the American Society of Nephrology.
Figures


Comment in
-
Glomerular Disease Pathology in the Era of Proteomics: From Pattern to Pathogenesis.J Am Soc Nephrol. 2018 Jan;29(1):2-4. doi: 10.1681/ASN.2017080881. Epub 2017 Nov 2. J Am Soc Nephrol. 2018. PMID: 29097622 Free PMC article. No abstract available.
References
-
- Fogo A, Qureshi N, Horn RG: Morphologic and clinical features of fibrillary glomerulonephritis versus immunotactoid glomerulopathy. Am J Kidney Dis 22: 367–377, 1993 - PubMed
-
- Rosenstock JL, Markowitz GS, Valeri AM, Sacchi G, Appel GB, D’Agati VD: Fibrillary and immunotactoid glomerulonephritis: Distinct entities with different clinical and pathologic features. Kidney Int 63: 1450–1461, 2003 - PubMed
-
- Iskandar SS, Falk RJ, Jennette JC: Clinical and pathologic features of fibrillary glomerulonephritis. Kidney Int 42: 1401–1407, 1992 - PubMed
-
- Markowitz GS, Cheng JT, Colvin RB, Trebbin WM, D’Agati VD: Hepatitis C viral infection is associated with fibrillary glomerulonephritis and immunotactoid glomerulopathy. J Am Soc Nephrol 9: 2244–2252, 1998 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

