How to rewire the host cell: A home improvement guide for intracellular bacteria
- PMID: 29097627
- PMCID: PMC5716269
- DOI: 10.1083/jcb.201701095
How to rewire the host cell: A home improvement guide for intracellular bacteria
Abstract
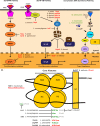
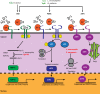
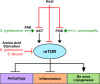
Intracellular bacterial pathogens have developed versatile strategies to generate niches inside the eukaryotic cells that allow them to survive and proliferate. Making a home inside the host offers many advantages; however, intracellular bacteria must also overcome many challenges, such as disarming innate immune signaling and accessing host nutrient supplies. Gaining entry into the cell and avoiding degradation is only the beginning of a successful intracellular lifestyle. To establish these replicative niches, intracellular pathogens secrete various virulence proteins, called effectors, to manipulate host cell signaling pathways and subvert host defense mechanisms. Many effectors mimic host enzymes, whereas others perform entirely novel enzymatic functions. A large volume of work has been done to understand how intracellular bacteria manipulate membrane trafficking pathways. In this review, we focus on how intracellular bacterial pathogens target innate immune signaling, the unfolded protein response, autophagy, and cellular metabolism and exploit these pathways to their advantage. We also discuss how bacterial pathogens can alter host gene expression by directly modifying histones or hijacking the ubiquitination machinery to take control of several host signaling pathways.
© 2017 Cornejo et al.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

