TDP-43 accelerates age-dependent degeneration of interneurons
- PMID: 29097807
- PMCID: PMC5668320
- DOI: 10.1038/s41598-017-14966-w
TDP-43 accelerates age-dependent degeneration of interneurons
Abstract
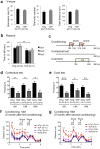
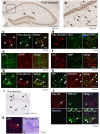
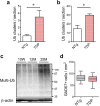
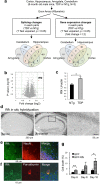
TDP-43 is an RNA-binding protein important for many aspects of RNA metabolism. Abnormal accumulation of TDP-43 in the cytoplasm of affected neurons is a pathological hallmark of the neurodegenerative diseases frontotemporal dementia (FTD) and amyotrophic lateral sclerosis (ALS). Several transgenic mouse models have been generated that recapitulate defects in TDP-43 accumulation, thus causing neurodegeneration and behavioural impairments. While aging is the key risk factor for neurodegenerative diseases, the specific effect of aging on phenotypes in TDP-43 transgenic mice has not been investigated. Here, we analyse age-dependent changes in TDP-43 transgenic mice that displayed impaired memory. We found the accumulation of abundant poly-ubiquitinated protein aggregates in the hippocampus of aged TDP-43 transgenic mice. Intriguingly, the aggregates contained some interneuron-specific proteins such as parvalbumin and calretinin, suggesting that GABAergic interneurons were degenerated in these mice. The abundance of aggregates significantly increased with age and with the overexpression of TDP-43. Gene array analyses in the hippocampus and other brain areas revealed dysregulation in genes linked to oxidative stress and neuronal function in TDP-43 transgenic mice. Our results indicate that the interneuron degeneration occurs upon aging, and TDP-43 accelerates age-dependent neuronal degeneration, which may be related to the impaired memory of TDP-43 transgenic mice.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

