Correlation of Somatostatin Receptor-2 Expression with Gallium-68-DOTA-TATE Uptake in Neuroblastoma Xenograft Models
- PMID: 29097943
- PMCID: PMC5612706
- DOI: 10.1155/2017/9481276
Correlation of Somatostatin Receptor-2 Expression with Gallium-68-DOTA-TATE Uptake in Neuroblastoma Xenograft Models
Abstract
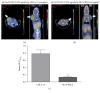
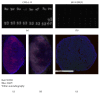
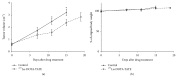
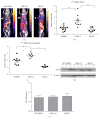
Peptide-receptor imaging and therapy with radiolabeled somatostatin analogs such as 68Ga-DOTA-TATE and 177Lu-DOTA-TATE have become an effective treatment option for SSTR-positive neuroendocrine tumors. The purpose of this study was to evaluate the correlation of somatostatin receptor-2 (SSTR2) expression with 68Ga-DOTA-TATE uptake and 177Lu-DOTA-TATE therapy in neuroblastoma (NB) xenograft models. We demonstrated variable SSTR2 expression profiles in eight NB cell lines. From micro-PET imaging and autoradiography, a higher uptake of 68Ga-DOTA-TATE was observed in SSTR2 high-expressing NB xenografts (CHLA-15) compared to SSTR2 low-expressing NB xenografts (SK-N-BE(2)). Combined autoradiography-immunohistochemistry revealed histological colocalization of SSTR2 and 68Ga-DOTA-TATE uptake in CHLA-15 tumors. With a low dose of 177Lu-DOTA-TATE (20 MBq/animal), tumor growth inhibition was achieved in the CHLA-15 high SSTR2 expressing xenograft model. Although, in vitro, NB cells showed variable expression levels of norepinephrine transporter (NET), a molecular target for 131I-MIBG therapy, low 123I-MIBG uptake was observed in all selected NB xenografts. In conclusion, SSTR2 expression levels are associated with 68Ga-DOTA-TATE uptake and antitumor efficacy of 177Lu-DOTA-TATE. 68Ga-DOTA-TATE PET is superior to 123I-MIBG SPECT imaging in detecting NB tumors in our model. Radiolabeled DOTA-TATE can be used as an agent for NB tumor imaging to potentially discriminate tumors eligible for 177Lu-DOTA-TATE therapy.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
