Circadian and Metabolic Effects of Light: Implications in Weight Homeostasis and Health
- PMID: 29097992
- PMCID: PMC5653694
- DOI: 10.3389/fneur.2017.00558
Circadian and Metabolic Effects of Light: Implications in Weight Homeostasis and Health
Abstract
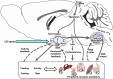
Daily interactions between the hypothalamic circadian clock at the suprachiasmatic nucleus (SCN) and peripheral circadian oscillators regulate physiology and metabolism to set temporal variations in homeostatic regulation. Phase coherence of these circadian oscillators is achieved by the entrainment of the SCN to the environmental 24-h light:dark (LD) cycle, coupled through downstream neural, neuroendocrine, and autonomic outputs. The SCN coordinate activity and feeding rhythms, thus setting the timing of food intake, energy expenditure, thermogenesis, and active and basal metabolism. In this work, we will discuss evidences exploring the impact of different photic entrainment conditions on energy metabolism. The steady-state interaction between the LD cycle and the SCN is essential for health and wellbeing, as its chronic misalignment disrupts the circadian organization at different levels. For instance, in nocturnal rodents, non-24 h protocols (i.e., LD cycles of different durations, or chronic jet-lag simulations) might generate forced desynchronization of oscillators from the behavioral to the metabolic level. Even seemingly subtle photic manipulations, as the exposure to a "dim light" scotophase, might lead to similar alterations. The daily amount of light integrated by the clock (i.e., the photophase duration) strongly regulates energy metabolism in photoperiodic species. Removing LD cycles under either constant light or darkness, which are routine protocols in chronobiology, can also affect metabolism, and the same happens with disrupted LD cycles (like shiftwork of jetlag) and artificial light at night in humans. A profound knowledge of the photic and metabolic inputs to the clock, as well as its endocrine and autonomic outputs to peripheral oscillators driving energy metabolism, will help us to understand and alleviate circadian health alterations including cardiometabolic diseases, diabetes, and obesity.
Keywords: desynchronization; metabolism; obesity; photic entrainment; suprachiasmatic nucleus.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

