Role of cytochrome P450 2J2 on cell proliferation and resistance to an anticancer agent in hepatocellular carcinoma HepG2 cells
- PMID: 29098037
- PMCID: PMC5652246
- DOI: 10.3892/ol.2017.6846
Role of cytochrome P450 2J2 on cell proliferation and resistance to an anticancer agent in hepatocellular carcinoma HepG2 cells
Abstract
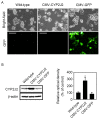
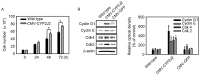
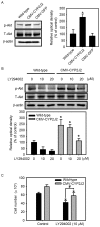
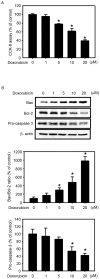
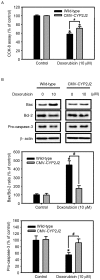
The present study examined the role of human cytochrome P450 2J2 (CYP2J2) on cell proliferation and resistance to an anticancer agent using stable hepatocellular carcinoma HepG2 cells overexpressing CYP2J2. Overexpression of CYP2J2 significantly increased HepG2 cell proliferation and the expression levels of cell cycle regulatory proteins, including cyclin D1, cyclin E, cyclin-dependent kinase (Cdk)2 and Cdk4. CYP2J2-overexpressing HepG2 cells exhibited high levels of Akt phosphorylation compared with those observed in wild-type HepG2 cells. Although Akt phosphorylation in both cell lines was significantly attenuated by LY294002, a specific phosphoinositide 3-kinase/Akt signaling inhibitor, the levels of Akt phosphorylation following treatment with LY294002 were higher in CYP2J2-overexpressing HepG2 cells than in wild-type HepG2 cells. Cell counting revealed that proliferation was reduced by LY294002 in both cell lines; however, CYP2J2-overexpressing HepG2 cell numbers were higher than those of wild-type HepG2 cells following treatment with LY294002. These results indicated that increased cell proliferation by CYP2J2 overexpression is mediated by increased Akt activity. It was also demonstrated that doxorubicin, an anticancer agent, reduced cell viability, induced a significant increase in the B-cell lymphoma (Bcl)-2 associated X protein (Bax)/Bcl-2 ratio and decreased pro-caspase-3 levels in wild-type HepG2 cells. However, the doxorubicin-induced reduction in cell viability was significantly attenuated by enhanced upregulation of CYP2J2 expression. The increase in the Bax/Bcl-2 ratio and the decrease in pro-caspase-3 levels were also recovered by CYP2J2 overexpression. In conclusion, CYP2J2 serves important roles in cancer cell proliferation and resistance to the anticancer agent doxorubicin in HepG2 cells.
Keywords: CYP2J2; HepG2 cells; cancer; proliferation; resistance.
Figures
References
-
- Capdevila JH, Falck JR, Harris RC. Cytochrome P450 and arachidonic acid bioactivation. Molecular and functional properties of the arachidonate monooxygenase. J Lipid Res. 2000;41:163–181. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
