Immunodeficiency in Bloom's Syndrome
- PMID: 29098565
- PMCID: PMC5742600
- DOI: 10.1007/s10875-017-0454-y
Immunodeficiency in Bloom's Syndrome
Abstract
Bloom's syndrome (BS) is an autosomal recessive disease, caused by mutations in the BLM gene. This gene codes for BLM protein, which is a helicase involved in DNA repair. DNA repair is especially important for the development and maturation of the T and B cells. Since BLM is involved in DNA repair, we aimed to study if BLM deficiency affects T and B cell development and especially somatic hypermutation (SHM) and class switch recombination (CSR) processes. Clinical data of six BS patients was collected, and immunoglobulin serum levels were measured at different time points. In addition, we performed immune phenotyping of the B and T cells and analyzed the SHM and CSR in detail by analyzing IGHA and IGHG transcripts using next-generation sequencing. The serum immunoglobulin levels were relatively low, and patients had an increased number of infections. The absolute number of T, B, and NK cells were low but still in the normal range. Remarkably, all BS patients studied had a high percentage (20-80%) of CD4+ and CD8+ effector memory T cells. The process of SHM seems normal; however, the Ig subclass distribution was not normal, since the BS patients had more IGHG1 and IGHG3 transcripts. In conclusion, BS patients have low number of lymphocytes, but the immunodeficiency seems relatively mild since they have no severe or opportunistic infections. Most changes in the B cell development were seen in the CSR process; however, further studies are necessary to elucidate the exact role of BLM in CSR.
Keywords: DNA repair; Immunodeficiency; bloom’s syndrome; class switch recombination; lymphocyte; somatic hypermutations.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
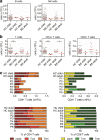
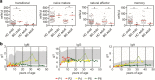
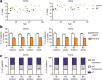

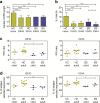
References
-
- Sanz MM, German J, Cunniff C. Bloom’s syndrome. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Mefford HC, Stephens K, Amemiya A, Ledbetter N, editors. GeneReviews® [Internet]. Seattle: University of Washington; 1993.
-
- Bloom D. Congenital telangiectatic erythema resembling lupus erythematosus in dwarfs; probably a syndrome entity. AMA Am J Dis Child. 1954;88(6):754–758. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

