Diaryl ethers with carboxymethoxyphenacyl motif as potent HIV-1 reverse transcriptase inhibitors with improved solubility
- PMID: 29098886
- PMCID: PMC6009982
- DOI: 10.1080/14756366.2017.1387542
Diaryl ethers with carboxymethoxyphenacyl motif as potent HIV-1 reverse transcriptase inhibitors with improved solubility
Abstract
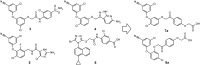
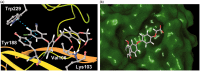
In search of new non-nucleoside reverse transcriptase inhibitors (NNRTIs) with improved solubility, two series of novel diaryl ethers with phenacyl moiety were designed and evaluated for their HIV-1 reverse transcriptase inhibition potentials. All compounds exhibited good to excellent results with IC50 at low micromolar to submicromolar concentrations. Two most active compounds (7e and 7 g) exhibit inhibitory potency comparable or even better than that of nevirapine and rilpivirine. Furthermore, SupT1 and CD4+ cell infectivity assays for the most promising (7e) have confirmed its strong antiviral potential while docking studies indicate a novel binding interactions responsible for high activity.
Keywords: HIV; NNRTI; drug solubility; reverse transcriptase.
Figures





References
-
- Autran B, Carcelain G, Li TS, et al. . Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science 1997;277:112–6. - PubMed
-
- De Clercq E. The role of non-nucleoside reverse transcriptase inhibitors (NNRTIs) in the therapy of HIV-1 infection. Antiviral Res 1998;38:153–79. - PubMed
-
- Mocroft A, Ledergerber B, Katlama C, et al. . Decline in the AIDS and death rates in the EuroSIDA study: an observational study. Lancet 2003;362:22–9. - PubMed
-
- de Béthune MP. Non-nucleoside reverse transcriptase inhibitors (NNRTIs), their discovery, development, and use in the treatment of HIV-1 infection: a review of the last 20 years (1989–2009). Antiviral Res 2010;85:75–90. - PubMed
-
- Hammer SM, Eron JJ Jr, Reiss P, et al. . Antiretroviral treatment of adult HIV infection: 2008 recommendations of the International AIDS Society-USA panel. J Am Med Assoc 2008;300:555–70. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
