Mechano-adaptation of the stem cell nucleus
- PMID: 29099288
- PMCID: PMC5973258
- DOI: 10.1080/19491034.2017.1371398
Mechano-adaptation of the stem cell nucleus
Abstract
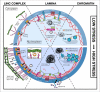
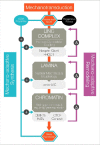
Exogenous mechanical forces are transmitted through the cell and to the nucleus, initiating mechanotransductive signaling cascades with profound effects on cellular function and stem cell fate. A growing body of evidence has shown that the force sensing and force-responsive elements of the nucleus adapt to these mechanotransductive events, tuning their response to future mechanical input. The mechanisms underlying this "mechano-adaptation" are only just beginning to be elucidated, and it remains poorly understood how these components act and adapt in tandem to drive stem cell differentiation. Here, we review the evidence on how the stem cell nucleus responds and adapts to physical forces, and provide a perspective on how this mechano-adaptation may function to drive and enforce stem cell differentiation.
Keywords: Epigenetics; Heterochromatin; LINC Complex; Lamin A/C; Mechanotransduction; Nuclear Mechanics; Stem Cells.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
