Vitamin D supplementation for chronic liver diseases in adults
- PMID: 29099543
- PMCID: PMC6485973
- DOI: 10.1002/14651858.CD011564.pub2
Vitamin D supplementation for chronic liver diseases in adults
Update in
-
Vitamin D supplementation for chronic liver diseases in adults.Cochrane Database Syst Rev. 2021 Aug 25;8(8):CD011564. doi: 10.1002/14651858.CD011564.pub3. Cochrane Database Syst Rev. 2021. PMID: 34431511 Free PMC article.
Abstract
Background: Vitamin D deficiency is often reported in people with chronic liver diseases. Therefore, improving vitamin D status could have a beneficial effect on people with chronic liver diseases.
Objectives: To assess the beneficial and harmful effects of vitamin D supplementation in people with chronic liver diseases.
Search methods: We searched The Cochrane Hepato-Biliary Group Controlled Trials Register, Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, Science Citation Index Expanded, and Conference Proceedings Citation Index - Science. We also searched databases of ongoing trials and the World Health Organization International Clinical Trials Registry Platform. We scanned bibliographies of relevant publications and asked experts and pharmaceutical companies for additional trials. All searches were up to January 2017.
Selection criteria: Randomised clinical trials that compared vitamin D at any dose, duration, and route of administration versus placebo or no intervention in adults with chronic liver diseases. Vitamin D could have been administered as supplemental vitamin D (vitamin D3 (cholecalciferol) or vitamin D2 (ergocalciferol)), or an active form of vitamin D (1α-hydroxyvitamin D (alfacalcidol), 25-hydroxyvitamin D (calcidiol), or 1,25-dihydroxyvitamin D (calcitriol)).
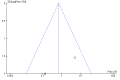
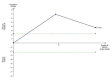
Data collection and analysis: We used standard methodological procedures expected by The Cochrane Collaboration. We contacted authors of the trials to ask for missing information. We conducted random-effects and fixed-effect meta-analyses. For dichotomous outcomes, we calculated risk ratios (RRs), and for continuous outcomes, we calculated mean differences (MD), both with 95% confidence intervals (CI) and Trial Sequential Analyses-adjusted CIs. We calculated Peto odds ratio (OR) for rare events. We considered risk of bias in domains to assess the risk of systematic errors. We conducted Trial Sequential Analyses to control the risk of random errors. We assessed the quality of the evidence with GRADE.
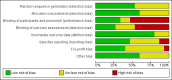
Main results: We included 15 randomised clinical trials with 1034 participants randomised. All trials had a parallel group design. Nine trials were conducted in high-income countries and six trials in middle-income countries. All trials were at high risk of bias. Six trials included participants with chronic hepatitis C, four trials included participants with liver cirrhosis, four trials included participants with non-alcoholic fatty liver disease, and one trial included liver transplant recipients. All included trials reported the baseline vitamin D status of participants. Participants in six trials had baseline 25-hydroxyvitamin D levels at or above vitamin D adequacy (20 ng/mL), while participants in the remaining nine trials were vitamin D insufficient (less than 20 ng/mL). All trials administered vitamin D orally. Mean duration of vitamin D supplementation was 0.5 years and follow-up was 0.6 years. Eleven trials (831 participants; 40% women; mean age 52 years) tested vitamin D3, one trial (18 men; mean age 61 years) with three intervention groups tested vitamin D2 and 25-dihydroxyvitamin D in separate groups, and three trials (185 participants; 55% women; mean age 55 years) tested 1,25-dihydroxyvitamin D. Seven trials used placebo, and eight trials used no intervention in the control group.The effect of vitamin D on all-cause mortality at the end of follow-up is uncertain because the results were imprecise (Peto OR 0.70, 95% CI 0.09 to 5.38; I2 = 32%; 15 trials; 1034 participants; very low quality evidence). Trial Sequential Analysis on all-cause mortality was performed based on a mortality rate in the control group of 10%, a relative risk reduction of 28% in the experimental intervention group, a type I error of 2.5%, and type II error of 10% (90% power). There was no diversity. The required information size was 6396 participants. The cumulative Z-curve did not cross the trial sequential monitoring boundary for benefit or harm after the 15th trial, and the Trial Sequential Analyses-adjusted CI was 0.00 to 2534.The effect of vitamin D on liver-related mortality (RR 1.62, 95% CI 0.08 to 34.66; 1 trial; 18 participants) and on serious adverse events such as hypercalcaemia (RR 5.00, 95% CI 0.25 to 100.8; 1 trial; 76 participants), myocardial infarction (RR 0.75, 95% CI 0.08 to 6.81; 2 trials; 86 participants), and thyroiditis (RR 0.33 95% CI 0.01 to 7.91; 1 trial; 68 participants) is uncertain because the results were imprecise. The evidence on all these outcomes is of very low quality. The effect of vitamin D3 on non-serious adverse events such as glossitis (RR 3.70, 95% CI 0.16 to 87.6; 1 trial; 65 participants; very low quality of evidence) is uncertain because the result was imprecise.Due to few data, we did not conduct Trial Sequential Analysis on liver-related mortality, and serious and non-serious adverse events.We found no data on liver-related morbidity and health-related quality of life in the randomised trials included in this review.
Authors' conclusions: We are uncertain as to whether vitamin D supplements in the form of vitamin D3, vitamin D2, 1,25-dihydroxyvitamin D, or 25-dihydroxyvitamin D have important effect on all-cause mortality, liver-related mortality, or on serious or non-serious adverse events because the results were imprecise. There is no evidence on the effect of vitamin D supplementation on liver-related morbidity and health-related quality of life. Our conclusions are based on few trials with an insufficient number of participants and on lack of data on clinically important outcomes. In addition, the analysed trials are at high risk of bias with significant intertrial heterogeneity. The overall quality of evidence is very low.
Conflict of interest statement
None known.
Figures




References
References to studies included in this review
Abu‐Mouch 2011 {published data only}
Atsukawa 2016 {published data only}
-
- Atsukawa M, Tsubota A, Shimada N, Yoshizawa K, Abe H, Asano T, et al. Effect of native vitamin D3 supplementation on refractory chronic hepatitis C patients in simeprevir with pegylated interferon/ribavirin. Hepatology Research 2016;46(5):450‐8. - PubMed
Barchetta 2016 {published data only}
Esmat 2015 {published data only}
-
- Esmat G, Raziky M, Elsharkawy A, Sabry D, Hassany M, Ahmed A, et al. Impact of vitamin D supplementation on sustained virological response in chronic hepatitis C genotype 4 patients treated by pegylated interferon/ribavirin. Journal of Interferon & Cytokine Research 2015;35(1):49‐54. - PubMed
Foroughi 2016 {published data only}
Lorvand Amiri 2016 {published data only}
-
- Lorvand Amiri H, Agah S, Mousavi SN, Hosseini AF, Shidfar F. Regression of non‐alcoholic fatty liver by vitamin D supplement: a double‐blind randomized controlled clinical trial. Archives of Iranian Medicine 2016;19(9):631‐8. - PubMed
-
- Lorvand Amiri H, Agah S, Tolouei Azar J, Hosseini S, Shidfar F, Mousavi SN. Effect of daily calcitriol supplementation with and without calcium on disease regression in non‐alcoholic fatty liver patients following an energy‐restricted diet: randomized, controlled, double‐blind trial. Clinical Nutrition 2016;16:31260‐2. - PubMed
Mobarhan 1984 {published data only}
-
- Mobarhan SA, Russell RM, Recker RR, Posner DB, Iber FL, Miller P. Metabolic bone disease in alcoholic cirrhosis: a comparison of the effect of vitamin D2, 25‐hydroxyvitamin D, or supportive treatment. Hepatology 1984;4(2):266‐73. - PubMed
Nimer 2012 {published data only}
Pilz 2016 {published data only}
Sharifi 2014 {published data only}
-
- Sharifi N, Amani R, Hajiani E, Cheraghian B. Does vitamin D improve liver enzymes, oxidative stress, and inflammatory biomarkers in adults with non‐alcoholic fatty liver disease? A randomized clinical trial. Endocrine 2014;47(1):70‐80. - PubMed
Shiomi 1999a {published data only}
-
- Shiomi S, Masaki K, Habu D, Takeda T, Nishiguchi S, Kuroki T, et al. Calcitriol for bone disease in patients with cirrhosis of the liver. Journal of Gastroenterology and Hepatology 1999;14(6):547‐52. - PubMed
Shiomi 1999b {published data only}
-
- Shiomi S, Masaki K, Habu D, Takeda T, Nishiguchi S, Kuroki T, et al. Calcitriol for bone loss in patients with primary biliary cirrhosis. Journal of Gastroenterology 1999;34(2):241‐5. - PubMed
Vosoghinia 2016 {published data only}
-
- Vosoghinia H, Esmaeilzadeh A, Ganji A, Hosseini SM, Jamehdar SA, Salehi M, et al. Vitamin D in standard HCV regimen (PEG‐Interferon plus Ribavirin), its effect on the early virologic r response rate: a clinical trial. Razavi Int J Med 2016;4(2):e36632.
Xing 2013 {published data only}
-
- Xing T, Qiu G, Zhong L, Ling L, Huang L, Peng Z. Calcitriol reduces the occurrence of acute cellular rejection of liver transplants: a prospective controlled study. Pharmazie 2013;68(10):821‐6. - PubMed
Yokoyama 2014 {published data only}
-
- Yokoyama S, Takahashi S, Kawakami Y, Hayes CN, Kohno H, Kohno H, et al. Effect of vitamin D supplementation on pegylated interferon/ribavirin therapy for chronic hepatitis C genotype 1b: a randomized controlled trial. Journal of Viral Hepatitis 2014;21(5):348‐56. - PubMed
References to studies excluded from this review
Atsukawa 2013 {published data only}
Benetti 2008 {published data only}
-
- Benetti A, Crosignani A, Varenna M, Giussani CS, Allocca M, Zuin M, et al. Primary biliary cirrhosis is not an additional risk factor for bone loss in women receiving regular calcium and vitamin D supplementation: a controlled longitudinal study. Journal of Clinical Gastroenterology 2008;42(3):306‐11. - PubMed
Bitetto 2010 {published data only}
-
- Bitetto D, Fabris C, Fornasiere E, Pipan C, Fumolo E, Cussigh A, et al. Vitamin D supplementation improves response to antiviral treatment for recurrent hepatitis C. Transplant International 2011;24(1):43‐50. - PubMed
Fernández Fernández 2016 {published data only}
-
- Fernández Fernández N, Linares Torres P, Joáo Matias D, Jorquera Plaza F, Olcoz Goñi JL. Vitamin D deficiency in chronic liver disease, clinical‐epidemiological analysis and report after vitamin d supplementation [Déficit de vitamina D en la enfermedad hepática crónica, análisis clínico epidemiológico y tras aporte vitamínico]. Gastroenterologia y Hepatologia 2016;39(5):305‐10. - PubMed
Floreani 2007 {published data only}
-
- Floreani A, Carderi I, Ferrara F, Rizzotto ER, Luisetto G, Camozzi V, et al. A 4‐year treatment with clodronate plus calcium and vitamin D supplements does not improve bone mass in primary biliary cirrhosis. Digestive and Liver Disease 2007;39(6):544‐8. - PubMed
Kitson 2016 {published data only}
-
- Kitson MT, Pham A, Gordon A, Kemp W, Roberts SK. High‐dose vitamin D supplementation and liver histology in NASH. Gut 2016;65(4):717‐8. - PubMed
Kondo 2013 {published data only}
Ladero 2013 {published data only}
-
- Ladero JM, Torrejón MJ, Sánchez‐Pobre P, Suárez A, Cuenca F, Orden V, et al. Vitamin D deficiency and vitamin D therapy in chronic hepatitis C. Annals of Hepatology 2013;12(2):199‐204. - PubMed
Long 1978 {published data only}
Malham 2012 {published data only}
-
- Malham M, Peter Jørgensen S, Lauridsen AL, Ott P, Glerup H, Dahlerup JF. The effect of a single oral megadose of vitamin D provided as either ergocalciferol (D2) or cholecalciferol (D3) in alcoholic liver cirrhosis. European Journal of Gastroenterology & Hepatology 2012;24(2):172‐8. - PubMed
Papapostoli 2016 {published data only}
-
- Papapostoli I, Lammert F, Stokes CS. Effect of short‐term vitamin D correction on hepatic steatosis as quantified by controlled attenuation parameter (CAP). Journal of Gastrointestinal and Liver Diseases 2016;25(2):175‐81. - PubMed
Rode 2010 {published data only}
-
- Rode A, Fourlanos S, Nicoll A. Oral vitamin D replacement is effective in chronic liver disease [Fréquence du déficit en vitamine D et effet de la supplémentation orale au cours des maladies chroniques du foie]. Gastroentérologie Clinique et Biologique 2010;34(11):618‐20. - PubMed
Stokes 2016 {published data only}
-
- Stokes CS, Grünhage F, Baus C, Volmer DA, Wagenpfeil S, Riemenschneider M, et al. Vitamin D supplementation reduces depressive symptoms in patients with chronic liver disease. Clinical Nutrition (Edinburgh, Scotland) 2016;35(4):950‐7. - PubMed
References to ongoing studies
IRCT2016020326342N1 {published data only}
-
- Effectiveness of Vitamin D Supplementation on Severity of Cirrhosis Based on CHILD and MELD Scores in Patients with Decompensate Cirrhosis.. Ongoing study March 2016..
NCT02779465 {published data only}
-
- Study of Oral Vitamin D Treatment for the Prevention of Hepatocellular Carcinoma in Patients with Chronic Hepatitis B.. Ongoing study June 2016..
Additional references
Arteh 2010
-
- Arteh J, Narra S, Nair S. Prevalence of vitamin D deficiency in chronic liver disease. Digestive Diseases and Sciences 2010;55(9):2624‐8. - PubMed
Atef 2017
-
- Atef SH. Vitamin D assays in clinical laboratory: past, present and future challenges. Journal of Steroid Biochemistry and Molecular Biology 2017 Feb 24 [Epub ahead of print]. - PubMed
Autier 2014
-
- Autier P, Boniol M, Mullie P. Vitamin D status and ill health: a systematic review. The Lancet. Diabetes & Endocrinology 2014;2(1):76‐89. - PubMed
Autier 2016
-
- Autier P. Vitamin D status as a synthetic biomarker of health status. Endocrine 2016;51(2):201‐2. - PubMed
Begg 1994
-
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50(4):1088‐101. - PubMed
Bischoff‐Ferrari 2009
-
- Bischoff‐Ferrari H. Vitamin D: what is an adequate vitamin D level and how much supplementation is necessary. Best Practice & Research. Clinical Rheumatology 2009;23(6):789‐95. - PubMed
Bitetto 2011
-
- Bitetto D, Fabris C, Falleti E, Toniutto P. Vitamin D deficiency and HCV chronic infection: what comes first. Journal of Hepatology 2011;55(4):944‐5. - PubMed
Bitetto 2012
-
- Bitetto D, Fabris C, Fornasiere E, Pipan C, Fumolo E, Cussigh A, et al. Vitamin D supplementation improves response to antiviral treatment for recurrent hepatitis C. Transplant International 2012;24(1):43‐50. - PubMed
Bjelakovic 2014a
Bjelakovic 2014b
Bolland 2006
-
- Bolland MJ, Grey AB, Ames RW, Mason BH, Horne AM, Gamble GD, et al. Determinants of vitamin D status in older men living in a subtropical climate. Osteoporosis International 2006;17(12):1742‐8. - PubMed
Bolland 2014
-
- Bolland MJ, Grey A, Gamble GD, Reid IR. The effect of vitamin D supplementation on skeletal, vascular, or cancer outcomes: a trial sequential meta‐analysis. Lancet, Diabetes Endocrinology 2014;2(4):307‐20. - PubMed
Borenstein 2009
-
- Borenstein M, Hedges LV, Higgins T, Rothstein HR. Introduction to Meta‐Analysis. Chichester (UK): John Wiley & Sons, 2009.
Bradburn 2007
-
- Bradburn MJ, Deeks JJ, Berlin JA, Russell Localio A. Much ado about nothing: a comparison of the performance of meta‐analytical methods with rare events. Statistics in Medicine 2007;26:53‐77. - PubMed
Brok 2008
-
- Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta‐analyses. Journal of Clinical Epidemiology 2008;61(8):763‐9. - PubMed
Brok 2009
-
- Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta‐analyses may be inconclusive ‐ Trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta‐analyses. International Journal of Epidemiology 2009;38(1):287‐98. - PubMed
Cacopardo 2012
-
- Cacopardo B, Camma C, Petta S, Pinzone MR, Cappellani A, Zanghi A, et al. Diagnostic and therapeutical role of vitamin D in chronic hepatitis C virus infection. Frontiers in Bioscience (Elite Edition) 2012;4:1276‐86. - PubMed
Chan 2004
-
- Chan AW, Hróbjartsson A, Haahr MT, Gøtzsche PC, Altman DG. Empirical evidence for selective reporting of outcomes in randomized trials: comparison of protocols to published articles. JAMA 2004;291(20):2457‐65. - PubMed
Chen 2014
-
- Chen EQ, Shi Y, Tang H. New insight of vitamin D in chronic liver diseases. Hepatobiliary & Pancreatic Diseases International 2014;13(6):580‐5. - PubMed
Chiang 2011
-
- Chiang KC, Yeh CN, Chen MF, Chen TC. Hepatocellular carcinoma and vitamin D: a review. Journal of Gastroenterology and Hepatology 2011;26(11):1597‐603. - PubMed
Cholongitas 2012
-
- Cholongitas E, Theocharidou E, Goulis J, Tsochatzis E, Akriviadis E, Burroughs K. Review article: the extra‐skeletal effects of vitamin D in chronic hepatitis C infection. Alimentary Pharmacology & Therapeutics 2012;35(6):634‐46. - PubMed
Cohen 1960
-
- Cohen J. A coefficient of agreement for nominal scales. Educational and Psychological Measurement 1960;20:37‐46.
Corey 2012
Davis 2007
-
- Davis CD, Dwyer JT. The "sunshine vitamin": benefits beyond bone?. Journal of the National Cancer Institute 2007;99(21):1563‐5. - PubMed
Dawson‐Hughes 2005
-
- Dawson‐Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R. Estimates of optimal vitamin D status. Osteoporosis International 2005;16(7):713‐6. - PubMed
Dawson‐Hughes 2010
-
- Dawson‐Hughes B, Mithal A, Bonjour JP, Boonen S, Burckhardt P, Fuleihan GE, et al. IOF position statement: vitamin D recommendations for older adults. Osteoporosis International 2010;21(7):1151‐4. - PubMed
DeMets 1987
-
- DeMets DL. Methods for combining randomized clinical trials: strengths and limitations. Statistics in Medicine 1987;6(3):341‐50. - PubMed
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. - PubMed
Egger 1997
Elangovan 2017
-
- Elangovan H, Chahal S, Gunton JE. Vitamin D in liver disease: current evidence and potential directions. Biochimica et Biophysica Acta 2017;1863(4):907‐16. - PubMed
Eliades 2013
-
- Eliades M, Spyrou E, Agrawal N, Lazo M, Brancati FL, Potter JJ, et al. Meta‐analysis: vitamin D and non‐alcoholic fatty liver disease. Alimentary Pharmacology & Therapeutics 2013;38(3):246‐54. - PubMed
Eliades 2015
Farnik 2013
-
- Farnik H, Bojunga J, Berger A, Allwinn R, Waidmann O, Kronenberger B, et al. Low vitamin D serum concentration is associated with high levels of hepatitis B virus replication in chronically infected patients. Hepatology 2013;58(4):1270‐6. - PubMed
Finkelmeier 2014
-
- Finkelmeier F, Kronenberger B, Köberle V, Bojunga J, Zeuzem S, Trojan J, et al. Severe 25‐hydroxyvitamin D deficiency identifies a poor prognosis in patients with hepatocellular carcinoma ‐ a prospective cohort study. Alimentary Pharmacology & Therapeutics 2014;39(10):1204‐12. - PubMed
Finkelmeier 2015
Fortmann 2013
-
- Fortmann SP, Burda BU, Senger CA, Lin JS, Whitlock EP. Vitamin and mineral supplements in the primary prevention of cardiovascular disease and cancer: an updated systematic evidence review for the U.S. Preventive Services Task Force. Annals of Internal Medicine 2013;159(12):824‐34. - PubMed
Furukawa 2007
-
- Furukawa TA, Watanabe N, Omori IM, Montori VM, Guyatt GH. Association between unreported outcomes and effect size estimates in Cochrane meta‐analyses. JAMA 2007;297(5):468‐70. - PubMed
Garattini 2016
-
- Garattini S, Jakobsen JC, Wetterslev J, Bertelé V, Banzi R, Rath A, et al. Evidence‐based clinical practice: overview of threats to the validity of evidence and how to minimise them. European Journal of Internal Medicine 2016;32:13‐21. - PubMed
Geier 2011
-
- Geier A. Shedding new light on vitamin D and fatty liver disease. Journal of Hepatology 2011;55(2):273‐5. - PubMed
Gluud 2006
-
- Gluud C. The culture of designing hepato‐biliary randomised trials. Journal of Hepatology 2006;44(3):607‐15. [MEDLINE: ] - PubMed
Gluud 2007
-
- Gluud C, Brok J, Gong Y, Koretz RL. Hepatology may have problems with putative surrogate outcome measures. Journal of Hepatology 2007;46(4):734‐42. - PubMed
Gluud 2008
-
- Gluud C, Hilden J. Povl Heiberg's 1897 methodological study on the statistical method as an aid in therapeutic trials, 2008. www.jameslindlibrary.org/articles/povl‐heibergs‐1897‐methodological‐stud... (accessed 17 July 2017). - PubMed
Gluud 2017
-
- Gluud C, Nikolova D, Klingenberg SL. Cochrane Hepato‐Biliary Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)). 2017; Issue 1. Art. No.: LIVER.
GRADEpro [Computer program]
-
- GRADE Working Group, McMaster University. GRADEpro. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Grey 2010
-
- Grey A, Bolland M. Vitamin D: a place in the sun. Archives of Internal Medicine 2010;170(13):1099‐100. - PubMed
Guallar 2010
-
- Guallar E, Miller ER 3rd, Ordovas JM, Stranges S. Vitamin D supplementation in the age of lost innocence. Annals of Internal Medicine 2010;152(5):327‐9. - PubMed
Guañabens 2010
-
- Guañabens N, Parés A. Liver and bone. Archives of Biochemistry and Biophysics 2010;503(1):84‐94. - PubMed
Gutierrez 2011
Han 2013
-
- Han YP, Kong M, Zheng S, Ren Y, Zhu L, Shi H, et al. Vitamin D in liver diseases: from mechanisms to clinical trials. Journal of Gastroenterology and Hepatology 2013;28(Suppl 1):49‐55. - PubMed
Harvey 2012
Higgins 2002
-
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. - PubMed
Higgins 2003
Higgins 2011
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hilger 2014
-
- Hilger J, Friedel A, Herr R, Rausch T, Roos F, Wahl DA, et al. A systematic review of vitamin D status in populations worldwide. British Journal of Nutrition 2014;111(1):23‐45. - PubMed
Hoan 2016
Holick 2007
-
- Holick MF. Vitamin D deficiency. New England Journal of Medicine 2007;357(3):266‐81. - PubMed
Holick 2011
-
- Holick MF, Binkley NC, Bischoff‐Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. Journal of Clinical Endocrinology 2011;96(7):1911‐30. - PubMed
Hollis 1999
Hollis 2008
-
- Hollis BW. Measuring 25‐hydroxyvitamin D in a clinical environment: challenges and needs. American Journal of Clinical Nutrition 2008;88(2):507S‐10S. - PubMed
ICH‐GCP 1997
-
- International Committee on Harmonization. Code of Federal Regulations & Guidelines. Vol. 1, Philadelphia, US: Barnett International/PAREXEL 1997.
Ioannidis 2009
-
- Ioannidis JP. Adverse events in randomized trials: neglected, restricted, distorted, and silenced. Archives of Internal Medicine 2009;169(19):1737‐9. - PubMed
IOM 2011
-
- Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: National Academies Press, 2011. - PubMed
Iruzubieta 2014
Jakobsen 2014
Jakobsen 2017
Johnson 2007
-
- Johnson RT, Dickersin K. Publication bias against negative results from clinical trials: three of the seven deadly sins. Nature Clinical Practice. Neurology 2007;3(11):590‐1. - PubMed
Keus 2010
Kitson 2012
-
- Kitson MT, Roberts SK. D‐livering the message: the importance of vitamin D status in chronic liver disease. Journal of Hepatology 2012;57(4):897‐909. - PubMed
Kitson 2014
-
- Kitson MT, Sarrazin C, Toniutto P, Eslick GD, Roberts SK. Vitamin D level and sustained virologic response to interferon‐based antiviral therapy in chronic hepatitis C: a systematic review and meta‐analysis. Journal of Hepatology 2014;61(6):1247‐52. - PubMed
Kjaergard 2001
-
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. - PubMed
Konstantakis 2016
Kovesdy 2013
Kupferschmidt 2012
-
- Kupferschmidt K. Uncertain verdict as vitamin D goes on trial. Science 2012;337(6101):1476‐8. - PubMed
Kwok 2013
-
- Kwok RM, Torres DM, Harrison SA. Vitamin D and nonalcoholic fatty liver disease (NAFLD): is it more than just an association. Hepatology 2013;58(3):1166‐74. - PubMed
Lange 2013
Lesser 2007
Li 2013
-
- Li YJ, Tang YW, Shi YQ, Han S, Wang JB, Zhou XM, et al. Polymorphisms in the vitamin D receptor gene and risk of primary biliary cirrhosis: a meta‐analysis. Journal of Gastroenterology and Hepatology 2013;29(4):706‐15. - PubMed
Lim 2012
-
- Lim LY, Chalasani N. Vitamin D deficiency in patients with chronic liver disease and cirrhosis. Current Gastroenterology Reports 2012;14(1):67‐73. - PubMed
Lips 2004
-
- Lips P. Which circulating level of 25‐hydroxyvitamin D is appropriate. Journal of Steroid Biochemistry and Molecular Biology 2004;89‐90(1‐5):611‐4. - PubMed
Lips 2006
-
- Lips P. Vitamin D physiology. Progress in Biophysics and Molecular Biology 2006;92(1):4‐8. - PubMed
Lips 2010
-
- Lips P. Worldwide status of vitamin D nutrition. Journal of Steroid Biochemistry and Molecular Biology 2010;121(1‐2):297‐300. - PubMed
Lucas 2005
-
- Lucas JA, Bolland MJ, Grey AB, Ames RW, Mason BH, Horne AM, et al. Determinants of vitamin D status in older women living in a subtropical climate. Osteoporosis International 2005;16(12):1641‐8. - PubMed
Lundh 2017
Luong 2012
Luong 2013a
Luong 2013b
Luxon 2011
-
- Luxon BA. Bone disorders in chronic liver diseases. Current Gastroenterology Reports 2011;13(1):40‐8. - PubMed
Mahamid 2013
Malham 2011
Moher 1998
-
- Moher D, Pham B, Jones A, Cook DJ, Jadad A, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analysis. Lancet 1998;352(9128):609‐13. - PubMed
Moher 2009
Nordic Trial Alliance 2015
-
- Nordic Trial Alliance working group on transparency and registration. Transparency and registration in clinical research in the Nordic countries. 07 Group, 2015.
Oliveira 2017
-
- Oliveira KD, Buss C, Tovo CV. Is there an association between vitamin D and liver fibrosis in patients with chronic hepatitis C. Arquivos de Gastroenterologia 2017;54(1):57‐9. - PubMed
Paternostro 2017
Petta 2010
-
- Petta S, Cammà C, Scazzone C, Tripodo C, Marco V, Bono A, et al. Low vitamin D serum level is related to severe fibrosis and low responsiveness to interferon‐based therapy in genotype 1 chronic hepatitis C. Hepatology 2010;51(4):1158‐67. - PubMed
Putz‐Bankuti 2012
-
- Putz‐Bankuti C, Pilz S, Stojakovic T, Scharnagl H, Pieber TR, Trauner M, et al. Association of 25‐hydroxyvitamin D levels with liver dysfunction and mortality in chronic liver disease. Liver International 2012;32(5):845‐51. - PubMed
Reid 2014
-
- Reid IR, Bolland MJ, Grey A. Effects of vitamin D supplements on bone mineral density: a systematic review and meta‐analysis. Lancet 2014;383(9912):146‐55. - PubMed
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Royle 2003
-
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. - PubMed
SACN 2016
-
- Public Health England. SACN vitamin D and health report, 2016. www.gov.uk/government/publications/sacn‐vitamin‐d‐and‐health‐report (accessed 17 July 2017).
Savović 2012a
-
- Savović J, Jones H, Altman D, Harris R, Jűni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomised controlled trials: combined analysis of meta‐epidemiological studies. Health Technology Assessment 2012;16(35):1‐82. - PubMed
Savović 2012b
-
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Annals of Internal Medicine 2012;157(6):429‐38. - PubMed
Sayiner 2016
-
- Sayiner M, Koenig A, Henry L, Younossi ZM. Epidemiology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis in the united states and the rest of the world. Clinics in Liver Disease 2016;20(2):205‐14. - PubMed
Schulz 1995
-
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. Journal of the American Medical Association 1995;273(5):408‐12. - PubMed
Siersma 2007
-
- Siersma V, Als‐Nielsen B, Chen W, Hilden J, Gluud LL, Gluud C. Multivariable modelling for meta‐epidemiological assessment of the association between trial quality and treatment effects estimated in randomized clinical trials. Statistics in Medicine 2007;26(14):2745‐58. - PubMed
Skaaby 2014
-
- Skaaby T, Husemoen LL, Borglykke A, Jørgensen T, Thuesen BH, Pisinger C, et al. Vitamin D status, liver enzymes, and incident liver disease and mortality: a general population study. Endocrine 2014;47(1):213‐20. - PubMed
Skaaby 2016
-
- Skaaby T, Husemoen LL, Thuesen BH, Pisinger C, Hannemann A, Jørgensen T. Longitudinal associations between lifestyle and vitamin D: a general population study with repeated vitamin D measurements. Endocrine 2016;51:342‐50. - PubMed
Stokes 2013
-
- Stokes CS, Volmer DA, Grünhage F, Lammert F. Vitamin D in chronic liver disease. Liver International 2013;33(3):338‐52. - PubMed
Stokes 2014
-
- Stokes CS, Krawczyk M, Reichel C, Lammert F, Grünhage F. Vitamin D deficiency is associated with mortality in patients with advanced liver cirrhosis. European Journal of Clinical Investigation 2014;44(2):176‐83. - PubMed
Storebø 2015
-
- Storebø OJ, Krogh HB, Ramstad E, Moreira‐Maia CR, Holmskov M, Skoog M, et al. Methylphenidate for attention‐deficit/hyperactivity disorder in children and adolescents: Cochrane systematic review with meta‐analyses and trial sequential analyses of randomised clinical trials. BMJ 2015;351:h5203. - PMC - PubMed
Sweeting 2004
-
- Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta‐analyses of sparse data. Statistics in Medicine 2004;23:1351‐75. - PubMed
Thorlund 2009
-
- Thorlund K, Devereaux PJ, Wetterslev J, Guyatt G, Ioannidis JP, Thabane L, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta‐analyses. International Journal of Epidemiology 2009;38(1):276‐86. - PubMed
Thorlund 2011a
-
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User Manual for Trial Sequential Analysis (TSA). Copenhagen Trial Unit, Centre for Clinical Intervention Research, Copenhagen, Denmark 2011.
Thorlund 2011b
Trépo 2013
-
- Trépo E, Ouziel R, Pradat P, Momozawa Y, Quertinmont E, Gervy C, et al. Marked 25‐hydroxyvitamin D deficiency is associated with poor prognosis in patients with alcoholic liver disease. Journal of Hepatology 2013;59(2):344‐50. - PubMed
TSA 2017 [Computer program]
-
- Copenhagen Trial Unit. Trial Sequential Analysis, version 0.9.5.5. Copenhagen Trial Unit, 2017.
Tsiaras 2011
-
- Tsiaras WG, Weinstock MA. Factors influencing vitamin D status. Acta Dermato‐venereologica 2011;91(2):115‐24. - PubMed
Turner 2013
van Schoor 2011
-
- Schoor NM, Lips P. Worldwide vitamin D status. Best Practice & Research. Clinical Endocrinology & Metabolism 2011;25(4):671‐80. - PubMed
Villar 2013
Wang 2013
-
- Wang JB, Abnet CC, Chen W, Dawsey SM, Fan JH, Yin LY, et al. Association between serum 25(OH) vitamin D, incident liver cancer and chronic liver disease mortality in the Linxian Nutrition Intervention Trials: a nested case‐control study. British Journal of Cancer 2013;109(7):1997‐2004. - PMC - PubMed
Wang 2015
Wesley Pike 2005
-
- Wesley Pike J, Shevde NK. The vitamin D receptor. In: Feldman D, Wesley Pike J, Glorieux FH editor(s). Vitamin D. 2nd Edition. Amsterdam (Netherlands): Elsevier Academic Press, 2005:167‐91.
Wetterslev 2008
-
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. - PubMed
Wetterslev 2009
Wetterslev 2017
Williamson 2005
-
- Williamson PR, Gamble C, Altman DG, Hutton JL. Outcome selection bias in meta‐analysis. Statistical Methods in Medical Research 2005;14(5):515‐24. - PubMed
Wood 2008
Younossi 2016
-
- Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease ‐ meta‐analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64(1):73‐84. - PubMed
Zittermann 2014
-
- Zittermann A, Ernst JB, Gummert JF, Börgermann J. Vitamin D supplementation, body weight and human serum 25‐hydroxyvitamin D response: a systematic review. European Journal of Nutrition 2014;53(2):367‐74. - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

