Mucin Production and Hydration Responses to Mucopurulent Materials in Normal versus Cystic Fibrosis Airway Epithelia
- PMID: 29099608
- PMCID: PMC5821906
- DOI: 10.1164/rccm.201706-1139OC
Mucin Production and Hydration Responses to Mucopurulent Materials in Normal versus Cystic Fibrosis Airway Epithelia
Abstract
Rationale: Cystic fibrosis (CF) airways disease produces a mucoobstructive lung phenotype characterized by airways mucus plugging, epithelial mucous cell metaplasia/hyperplasia, chronic infection, and inflammation. Simultaneous biochemical and functional in vivo studies of mucin synthesis and secretion from CF airways are not available. In vitro translational models may quantitate differential CF versus normal mucin and fluid secretory responses to infectious/inflammatory stimuli.
Objectives: We tested the hypothesis that CF airways exhibit defective epithelial fluid, but not mucin, secretory responses to bacterial/inflammatory host products.
Methods: Well-differentiated primary human bronchial epithelial cultures were exposed to supernatant from mucopurulent material (SMM) from human CF airways as a test of bacterial/inflammatory host product stimulus. Human bronchial epithelia (HBE) with normal CF transmembrane conductance regulator function were compared with ΔF508/ΔF508 CF HBE.
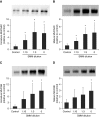
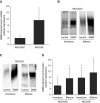
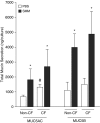
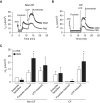
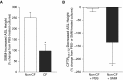
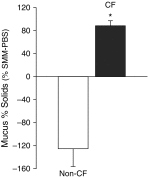
Measurements and main results: Acute (up to 60 min) SMM exposure promoted mucin secretion, but mucins were degraded by the proteolytic enzymes present in SMM. Chronic SMM exposure induced upregulation of mucin synthesis and storage and generated absolute increases in basal and stimulated mucin release in normal and CF cultures. These responses were similar in normal and CF cultures. In contrast, SMM produced a coordinated CF transmembrane conductance regulator-mediated Cl- secretory response in normal HBE, but not in CF HBE. The absence of the fluid secretory response in CF produced quantitatively more dehydrated mucus.
Conclusions: Our study reveals the interplay between regulation of mucin and fluid secretion rates in inflamed versus noninflamed conditions and why a hyperconcentrated mucus is produced in CF airways.
Keywords: airway hydration; airway inflammation; airway mucins; cystic fibrosis.
Figures
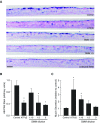
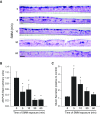
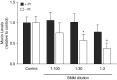
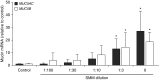
Comment in
-
Mucopurulent Triggering of the Airway Epithelium. Implications in Health and Cystic Fibrosis.Am J Respir Crit Care Med. 2018 Feb 15;197(4):418-420. doi: 10.1164/rccm.201712-2554ED. Am J Respir Crit Care Med. 2018. PMID: 29298399 No abstract available.
References
-
- Holmén JM, Karlsson NG, Abdullah LH, Randell SH, Sheehan JK, Hansson GC, et al. Mucins and their O-glycans from human bronchial epithelial cell cultures. Am J Physiol Lung Cell Mol Physiol. 2004;287:L824–L834. - PubMed
-
- Rose MC, Voynow JA. Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol Rev. 2006;86:245–278. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

