Hematopoietic Stem Cell Gene Therapy: Progress and Lessons Learned
- PMID: 29100011
- PMCID: PMC6039108
- DOI: 10.1016/j.stem.2017.10.010
Hematopoietic Stem Cell Gene Therapy: Progress and Lessons Learned
Abstract
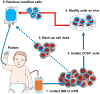
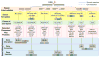
The use of allogeneic hematopoietic stem cells (HSCs) to treat genetic blood cell diseases has become a clinical standard but is limited by the availability of suitable matched donors and potential immunologic complications. Gene therapy using autologous HSCs should avoid these limitations and thus may be safer. Progressive improvements in techniques for genetic correction of HSCs, by either vector gene addition or gene editing, are facilitating successful treatments for an increasing number of diseases. We highlight the progress, successes, and remaining challenges toward the development of HSC gene therapies and discuss lessons they provide for the development of future clinical stem cell therapies.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures

References
-
- Aiuti A, Biasco L, Scaramuzza S, Ferrua F, Cicalese MP, Baricordi C, Dionisio F, Calabria A, Giannelli S, Castiello MC, Bosticardo M, Evangelio C, Assanelli A, Casiraghi M, Di Nunzio S, Callegaro L, Benati C, Rizzardi P, Pellin D, Di Serio C, Schmidt M, Kalle von C, Gardner J, Mehta N, Neduva V, Dow DJ, Galy A, Miniero R, Finocchi A, Metin A, Banerjee PP, Orange JS, Galimberti S, Valsecchi MG, Biffi A, Montini E, Villa A, Ciceri F, Roncarolo MG, Naldini L. Lentiviral Hematopoietic Stem Cell Gene Therapy in Patients with Wiskott-Aldrich Syndrome. Science. 2013;341:1233151–1233151. doi: 10.1126/science.1233151. - DOI - PMC - PubMed
-
- Aiuti A, Cattaneo F, Galimberti S, Benninghoff U, Cassani B, Callegaro L, Scaramuzza S, Andolfi G, Mirolo M, Brigida I, Tabucchi A, Carlucci F, Eibl M, Aker M, Slavin S, Al-Mousa H, Ghonaium Al A, Ferster A, Duppenthaler A, Notarangelo L, Wintergerst U, Buckley RH, Bregni M, Marktel S, Valsecchi MG, Rossi P, Ciceri F, Miniero R, Bordignon C, Roncarolo MG. Gene therapy for immunodeficiency due to adenosine deaminase deficiency. N Engl J Med. 2009;360:447–458. doi: 10.1056/NEJMoa0805817. - DOI - PubMed
Web resources
-
- bluebird bio Inc. 2017 Published online September 06, 2017 http://www.businesswire.com/news/home/20170906005645/en/
-
- European Medicines Agency. 2016 Published online April 01, 2016 http://www.ema.europa.eu/docs/en_GB/document_library/Press_release/2016/....
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

